Continuing Education Activity
Ciguatera toxicity is the most common nonbacterial, seafood-borne illness worldwide. While this condition is most prevalent in coastal areas, contaminated fish transported and sold in nonendemic regions still pose a health risk. Physicians everywhere should be aware of this disease and be familiar with its risks, symptoms, diagnosis, and treatment. Patients primarily present with gastrointestinal symptoms, but the condition may also have neurologic, cardiac, and dermatologic manifestations.
This activity for healthcare professionals is designed to enhance learners' proficiency in evaluating and managing ciguatera toxicity. This activity is designed to equip learners to collaborate effectively with an interprofessional team caring for patients affected by this condition.
Objectives:
Identify the pathophysiologic mechanisms that cause ciguatera toxicity symptoms.
Identify the constellation of signs and symptoms indicative of ciguatera poisoning and develop a clinically guided diagnostic plan for a patient suspected to have this condition.
Develop an individualized management plan for a patient diagnosed with ciguatera poisoning.
Implement interprofessional team coordination and communication strategies when formulating short- and long-term care plans for individuals diagnosed with ciguatera toxicity.
Introduction
Ciguatera poisoning is the most common nonbacterial seafood poisoning globally.[1] The condition arises after consuming fish contaminated with the lipophilic, heat-stable xenobiotic ciguatoxin. The toxin is produced by single-celled dinoflagellates in the Gambierdiscus and Fukuyoa genera. Dinoflagellates are dominant phytoplankton members and can be found in all oceans between 35° north and 35° south latitudes.[2] Ciguatera poisoning is endemic in tropical and subtropical waters.[3]
The ciguatoxin is initially introduced into the flesh of herbivorous fish through the consumption of ciguatoxin-producing dinoflagellates. The toxin accumulates through predation until it reaches the top-tier predatory fish in sufficient concentrations. Consumption of contaminated fish harms humans. The fish that most commonly cause ciguatera toxicity are barracuda, grouper, moray eel, amberjack, sea bass, sturgeon, parrotfish, surgeonfish, and red snapper.[4]
Ciguatoxin is a potent poison that may cause gastrointestinal, neurologic, cardiovascular, and dermatologic symptoms. While uncomfortable, ciguatera poisoning is rarely fatal.[5] Understanding the symptomatology of this condition is essential to clinicians everywhere. Symptom onset may be delayed for several days. Thus, travelers may consult for the condition after moving to a different location. Contaminated fish may also be marketed in nonendemic regions.[6][7]
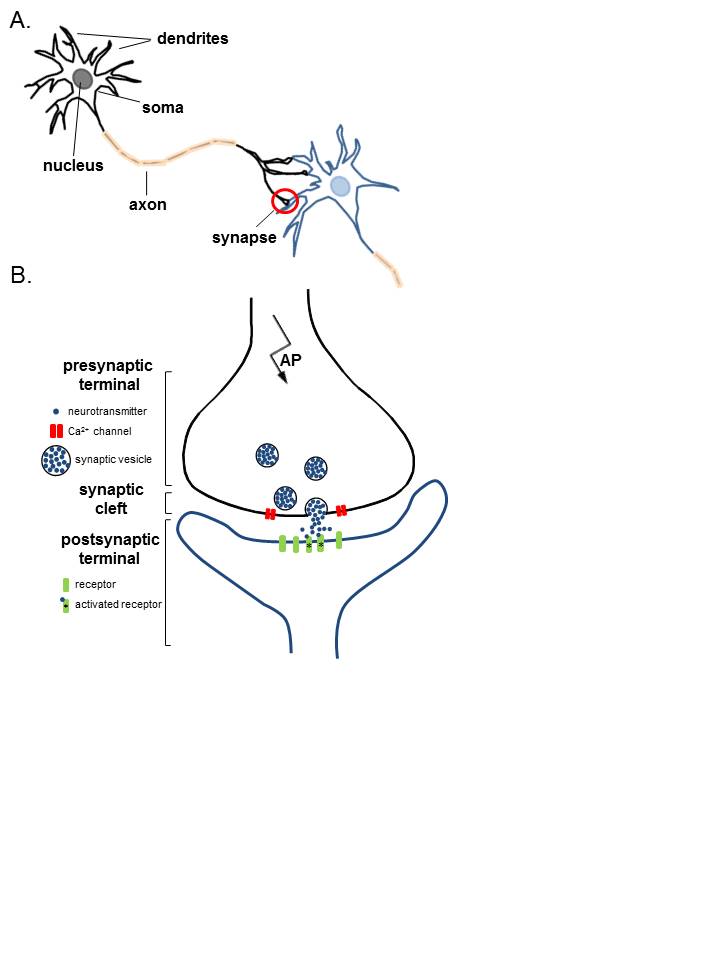
Anatomy of the Synapse
The typical motor neuron has 3 major parts: the soma, axon, and dendrites (see Image. Anatomy of Neurons). The soma is the neuron's body, containing most cellular organelles. The axon is an outgrowth extending from the soma to a peripheral nerve. The dendrites are the soma's branching projections extending to surrounding areas in the spinal cord. Dendrites can be 1 mm or shorter in length.
Presynaptic terminals are the axonal or dendritic tips resembling small round knobs that release neurotransmitters at the synaptic cleft. Transmitter vesicles and mitochondria lie in the presynaptic terminals. Transmitter vesicles contain the neurotransmitters diffusing through the synaptic cleft to reach the next (postsynaptic) neuron. The mitochondria provide the energy that enables neurotransmitter synthesis, release, and reuptake at the presynaptic terminal.
The presynaptic membrane covers the presynaptic terminal and contains most of the neurons' voltage-gated calcium channels. Neuronal depolarization opens these calcium channels, increasing presynaptic calcium concentration. Calcium ions bind to release sites on the inner surface of the presynaptic membrane, stimulating transmitter vesicle transport toward the membrane and the vesicles' eventual exocytosis. The vesicles then release neurotransmitters in the synaptic cleft.
Postsynaptic surfaces may be found on the dendrites, bodies, and axons. The postsynaptic membrane has receptors that neurotransmitters may stimulate or inhibit. Some receptors act as ion channels, allowing cation or anion entry on neurotransmitter activation. Cations like sodium and calcium excite neurons, while anions like chloride do the opposite. However, an increased concentration of potassium, another cation, can inhibit neurons. The effects of ion channels are short-lived but important for quick activities like sensation and motion. Meanwhile, other postsynaptic receptors act as second-messenger system activators, enabling long-term neuronal functions such as memory.
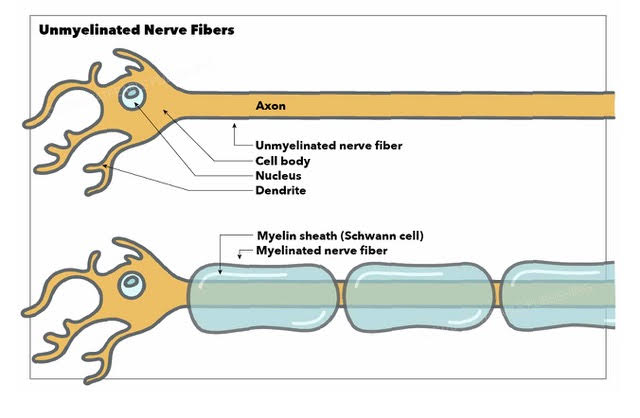
Nerve Fiber Classification
Spinal nerve fibers are either myelinated or unmyelinated (see Image. Unmyelinated and Myelinated Nerve Fibers). Myelination increases neuronal signal transmission rates.
Type A fibers are myelinated and further divided into Aα, Aβ, Aγ, and Aδ. Type Aα fibers have the largest diameter of all spinal nerve fibers and are the fastest signal transmitters. Type Aα fibers provide motor impulses to the skeletal muscles and serve as the afferent pathways for muscle spindles. Type Aβ fibers are thinner than type Aα but also innervate the skeletal muscles and muscle spindles. Types Aγ and Aδ are even thinner and slower than Aα and Aβ types and serve mostly sensory functions.
Type C fibers are unmyelinated and have the smallest diameters. Thus, these spinal nerve fibers transmit signals slowly. Half of the peripheral nerve sensory fibers and all postganglionic autonomic fibers are of the C type.
Spinal nerve fibers may also be classified into Groups Ia, Ib, II, III, and IV. Group Ia fibers are type Aα fibers in the muscle spindles' annulospiral tips. Group Ib fibers are type Aα fibers supplying the Golgi tendon organs.
Group II fibers innervate cutaneous tactile receptors and the muscle spindles' flower-spray endings. These fibers are either type Aβ or Aγ. Group III fibers are of the Aδ type, carrying the sensations of temperature, crude touch, and pricking pain (fast pain). Group IV fibers are type C fibers that transmit "slow pain" (ie, visceral pain), itch, temperature, and crude touch sensations.
Etiology
Consuming ciguatoxin-contaminated seafood is mainly responsible for the manifestations of ciguatera poisoning. Warmer months, eg, May through August in the northern hemisphere, are associated with an increased risk of poisoning, as phytoplanktons proliferate the fastest during these months.[8][9] However, warmer ocean temperatures from climate change and increased water carbon dioxide content favor phytoplankton and algal bloom occurrences throughout the year. Thus, ciguatera poisoning outbreaks can occur even in relatively cooler seasons. Additionally, rising ocean temperatures have expanded ciguatera-affected regions by enabling the growth of ciguatoxin-carrying marine species in previously nonendemic sites.[10]
Due to bioaccumulation, tropical predator species are most likely to cause ciguatera toxicity. Barracudas, groupers, moray eels, snapper, and amberjacks are commonly implicated. However, ciguatera contamination has also been reported in over 400 reef fish species. Any part of the fish can contain the toxin, and concentrations of 0.1 parts per billion in the fish's flesh is enough to cause illness.[11] Ciguatoxin is tasteless, odorless, lipid-soluble, and heat-resistant. Therefore, the poison can evade the senses and cannot be easily destroyed by cooking contaminated food. People who consume seafood may not know if they have ingested the toxin unless they can connect the incident to publicly reported toxic algal blooms.[12]
Epidemiology
Ciguatera toxicity is the most common worldwide nonbacterial fish poisoning, with up to 50,000 to 500,000 cases occurring globally annually. This number is likely underreported, as most physicians are probably unaware that ciguatera poisoning is reportable. People's exposure risk may increase when highly contaminated fish visceral tissues are consumed.[13] Fortunately, the mortality rate of this condition is low at less than 1%.[14] However, symptoms typically last for weeks to months and sometimes years.[15]
Ciguatera poisoning is endemic to the South Pacific and Caribbean, though ciguatoxin contamination of marine species has also been detected in the Indian Ocean. Ciguatoxin has numerous types, but the most common are Pacific and Caribbean ciguatoxin-1. Pacific ciguatoxin-1 is 10 times more toxic than Caribbean ciguatoxin-1.
Pathophysiology
Ciguatoxin is a potent sodium channel poison in mammals. The toxin causes sustained sodium channel activation and blocks potassium channels. These actions combined lead to membrane depolarization and spontaneous, repetitive action potential firing (see Image. Sites and Modes of Action of Ciguatoxins).
Increased sodium influx damages excitable cells, particularly the neurons and cardiac myocytes. Swelling of the axons, nerve terminals, and perisynaptic Schwann cells occurs in the neurons. Meanwhile, in the cardiac myocytes, animal studies demonstrate that sodium channel-mediated ciguatoxin action influences cardiac contractility. Moderate ciguatoxin concentrations have indirect and direct positive inotropic actions, while high toxin levels produce negative inotropic effects.[16]
Ciguatoxin’s activation of calcium as a secondary messenger contributes to the cardiovascular, musculoskeletal, and gastrointestinal manifestations of the illness. Intracellular calcium increases due to inositol triphosphate-mediated stored calcium release, terminal calcium channel activation, and sodium-calcium exchanger stimulation. Consequently, spontaneous and tetanic contractions occur in the heart, skeletal, and smooth muscles. Intestinal smooth muscle contraction disturbances lead to intestinal fluid stimulation and diarrhea without mucosal injury. Synaptic vesicle recycling is also impaired, exhausting neurotransmitter reserves.
The toxin causes pruritus owing to deranged low-frequency discharge in C-polymodal nociceptive fibers (cutaneous afferent unmyelinated fibers), which are not normally active in undamaged skin at normal temperatures.[17] Cold allodynia or cold temperature inversion is due to increased nerve depolarization of type Aδ and C fibers.
Toxicokinetics
Ciguatoxin toxicokinetics have not been extensively studied. However, Pacific ciguatoxin-1 was investigated in a rat model. The peak serum concentration after oral ingestion was achieved after 2 hours. The half-life after oral ingestion was calculated to be 82 hours. Ciguatoxin activity remained in the liver, muscle, and brain 96 hours after intraperitoneal and oral dosing. Elimination was primarily fecal, with a small fraction cleared by the renal route.[18]
History and Physical
Ciguetera poisoning is a clinical diagnosis. A thorough history and physical examination are crucial to diagnostic accuracy and timeliness. No laboratory or radiologic studies are available to aid in diagnosis. The clinical symptoms of this condition include gastrointestinal and neurological effects that may start within 1 hour or upwards of 1 to 2 days after consuming contaminated fish.
Diarrhea is the most commonly reported symptom. However, patients may also present with other signs of gastrointestinal involvement, like abdominal pain, nausea, vomiting, gastric upset, and belching. Symptom onset is highly variable and may occur in as short as 30 minutes to 1 hour after ingestion or be delayed until 48 hours. About 90% of cases occur within 12 hours. Diarrhea lasts about 5 days on average.
Mouth, hand, and foot paresthesias are the predominant neurological manifestations of ciguatera poisoning. Other neurological symptoms include headache, ataxia, dizziness or vertigo, seizures, perioral paresthesia, numbness, metallic taste in the mouth, blurred vision, ataxia, pruritus, meningismus, tremors, and hallucinations. Severe ciguatera toxicity may present with cold allodynia or a burning sensation on contact with a cold object. Cold allodynia has other possible causes but is essentially pathognomonic of substance poisoning in the right setting.[19]
Dermatologic effects that have been reported are pruritus, urticaria, and bullous or desquamating skin effects.[20][21][22] Pruritus is present in 50-65% of cases and may be severe.[23] Pruritus may also be aggravated by ethanol use.[24]
Cardiovascular effects are rare but may include bradycardia, hypotension, and ectopic beats.[25][26][27] Patients must be asked about palpitations, positional dizziness, dyspnea, and lightheadedness, as the toxin can cause bradycardia and ventricular arrhythmia. However, cardiac symptoms are typically only present in the early stages of the toxicity and improve with time.[28]
Sexual and obstetrical complications include reports of dyspareunia in a female patient following sexual intercourse with a person known to have ciguatera toxicity. This occurrence suggests that the toxin may be transmitted through semen.[29] Ciguatoxin is not known to be teratogenic. However, the poison has been shown to cross the placental barrier and cause abnormal fetal movements.[30] Ciguatoxin thus increases the risk of preterm labor and spontaneous abortions.[27] Lastly, affected mothers have reported diarrhea and facial rashes in their breastfeeding infants.[31]
Musculoskeletal manifestations of ciguatera poisoning include myalgias and arthralgias. Dysuria, hypersalivation, and sleep disturbances may also be reported.
Most patients with ciguatera symptoms recover after a few days to weeks. However, some individuals may remain symptomatic for years. Alcohol consumption during toxin ingestion has been found to increase the risk of developing bradycardia, hypotension, and altered skin sensation. Relapses may be triggered by consuming alcohol, nuts, seeds, fish, chicken, and eggs.[32]
The patient's travel and social history and dietary patterns must be elicited during history taking. A detailed physical examination must be performed for every patient. Particular attention should be given to the exam's gastrointestinal, cardiac, neuromuscular, and dermatologic segments. Pelvic examination may be performed in individuals reporting urogenital symptoms.
Evaluation
Multiple tests can detect ciguatoxin, such as liquid chromatography-mass spectrometry, cytotoxicity assays, and receptor-binding immunoassays.[33][34] However, these tests are used more in an academic or research setting and are typically not clinically available. Additionally, these tests take days to yield results and are thus inappropriate for emergency department visits.
Routine diagnostic tests are often nonspecific. However, some tests are useful for monitoring ciguatoxin effects in the body. For example, metabolic panels may help determine fluid and electrolyte imbalances due to abdominal symptoms. Fecalysis may not show pathologic organisms. An electrocardiogram can help look for arrhythmia in patients with cardiovascular symptoms. Imaging tests can help rule out neurologic lesions if lateralizing neurologic signs are present on physical examination. The clinical presentation must guide the choice of diagnostic exams.
Treatment / Management
The treatment of ciguatera poisoning is primarily supportive, as the toxin does not have a specific antidote. Nausea and vomiting should be treated with ondansetron or another antiemetic. Antidiarrheals may also be considered to treat diarrhea. Dehydration can occur due to vomiting and diarrhea. Thus, patients should be treated with oral fluids or, if unable to take fluids by mouth, intravenous fluids.
Antihistamines and amitriptyline may be considered for pruritus. Cold showers may also temporarily relieve skin burning and pruritus. Non-steroidal anti-inflammatory medications can relieve musculoskeletal pain. Evidence suggests that gabapentin and amitriptyline may also help treat neuropathies associated with ciguatera poisoning.[35][36] Seizures should be treated with benzodiazepines. For refractory seizures, barbiturates or propofol may be considered.
Neuropathic symptoms may be exacerbated by alcohol or vigorous exercise. Therefore, patients should be advised to avoid alcohol and vigorous physical activities for at least several months.[30] Others that patients must avoid include fresh or preserved fish, fish sauces, shellfish and shellfish products, nuts, and nut oils. Symptomatic bradycardia is treated with intravenous atropine.[37] Direct α-adrenergic agonists like phenylephrine may be given for orthostasis.
If the patient presents before the onset of nausea and vomiting, activated charcoal may be used in the first few hours of ingestion to prevent further intestinal ciguatoxin absorption.[38] However, aspiration and pneumonitis are possible risks if vomiting starts after activated charcoal administration.
Intravenous mannitol is a controversial treatment. Mannitol is an osmotic diuretic that can, in theory, reduce neuronal edema and abnormal action potentials. Anecdotal evidence suggests that this agent can decrease neuropathic symptoms or prevent the onset of neurologic symptoms. However, a double-blinded trial demonstrated that intravenous mannitol offers no added benefit to patients with ciguatera toxicity compared to normal saline.[39] Meanwhile, some physicians advocate for the treatment, suggesting that mannitol's benefits outweigh the potential for harm and thus must be considered in acute ciguatera poisoning management.[40]
Differential Diagnosis
Diagnosing ciguatera poisoning is often difficult due to the symptoms' nonspecificity, relative rarity in non-coastal locations, clinicians' lack of familiarity with the illness, and absence of ciguatera-specific diagnostic tests. However, some of the conditions that resemble ciguatera poisoning include the following:
- Shellfish poisoning, which has the amnesic (domoic acid), diarrheic (okadaic acid), neurotoxic (brevetoxin), and paralytic (saxitoxin) forms and is also related to algal blooms
- Scombroid poisoning
- Common enteroviruses
- Organophosphate toxicity
- Botulism
- Multiple sclerosis
- Guillain-Barre syndrome
- Acute gastroenteritis from other sources like Salmonella, Escherichia coli, Entamoeba histolytica
A careful collection of history and physical examination findings are key to diagnosis. Laboratory exams may help rule out other causes.
Prognosis
Ciguatera toxicity is generally self-limiting, with symptoms typically lasting for a few days. However, a prolonged symptomatology duration is possible, with some patients experiencing symptoms for months to years. Predisposing factors to a lengthy illness course are currently unknown. However, patients should be counseled to avoid fish, caffeine, alcohol, and nuts within 6 months of poisoning, as these foods and beverages can trigger symptom recurrences. Mortality has only been reported in 1% of patients who have ciguatera toxicity.
Complications
Besides the previously described organ-specific manifestations, untreated ciguatera poisoning may cause dehydration, weakness, palpitations, temperature sensitivity, and psychological disturbances. Some individuals may experience symptom recurrence months or even years after the initial toxin exposure. Fatalities are very rare but have been reported. The prognosis of this condition is overall very good.
Consultations
Consultation for recommendations on testing and treatment may be accomplished by contacting the following authorities:
- Local poison center: via the Poison Help line at 1800-222-1222
- Centers for Disease Control: by phone at 1800-232-4636 or online at https://wwwn.cdc.gov/dcs/ContactUs/Form
- Local public health department
Specialists like cardiologists and neurologists may also be consulted for organ-specific manifestations.
Deterrence and Patient Education
Patients planning on traveling to ciguatera-endemic locations should be educated about the following to prevent toxin exposure:
- Marine toxins and possible food sources
- Watching out for public alerts about toxic algal blooms
- Proper fish handling and preparation, ie, removing the liver, skin, and scales—parts where ciguatoxin mostly accumulates— when eating a known high-risk fish
- Reminding people that ciguatoxin is heat-stable
- Seeking medical attention immediately if symptomatic to prevent complications
- Avoiding foods and beverages that can trigger recurrences
- Working with local authorities and healthcare providers to promote awareness of this condition
By following these preventive measures, individuals can reduce the risk of ciguatera poisoning and contribute to community efforts in promoting food safety.
Pearls and Other Issues
The most important points to remember when evaluating and managing ciguatera poisoning are the following:
- Ciguatera poisoning is a clinical diagnosis. The typical manifestations include gastrointestinal, neurologic, dermatologic, and cardiovascular symptoms. Muscular and genitourinary complaints may also be present. This condition must be considered in a patient who recently traveled to an endemic location or consumed seafood from an endemic region.
- No specific clinical tests are available for ciguatoxin detection. However, diagnostic tests may be obtained to assess for complications that may arise from the condition or rule out other diseases.
- Management is mainly supportive.
- The prognosis of ciguatera toxin is excellent overall, although some individuals may experience a protracted illness course.
- Alcohol, caffeine, fish, and nuts can trigger recurrence.
- The condition is best prevented by avoiding consuming seafood from endemic areas.
- Heat cannot easily destroy the poison.
Ciguatera poisoning can mimic other foodborne illnesses. A thorough clinical and diagnostic evaluation can help distinguish this condition from similar clinical entities.
Enhancing Healthcare Team Outcomes
Patients with ciguatera poisoning initially present to the emergency department. However, the lack of a rapid diagnostic test requires clinical acumen and a degree of suspicion to make the diagnosis. The poisoning is best managed by a team that includes the emergency department physician, infectious disease expert or medical toxicologist, internist, and primary care provider.
Cardiologists and neurologists may be involved in managing cardiac and neurologic symptoms, respectively. Toxicology pharmacists can inform patients about ciguatoxin and the pharmacologic treatments given as supportive measures. Nurses can monitor patient status, coordinate care, and provide updates to the team.
Effective communication and collaboration among these healthcare professionals ensure a holistic and well-coordinated approach to the assessment, management, and follow-up care for individuals affected by ciguatera poisoning.