Continuing Education Activity
Salmonellae are motile gram-negative bacilli that infect or colonize humans, causing a number of clinical infections with different clinical features like gastroenteritis, enteric fever, bacteremia, and a chronic carrier state. Enteric fever is caused by Salmonella typhi and Salmonella paratyphi, while other strains of Salmonella are known as nontyphoidal strains. This activity reviews the evaluation and management of Salmonella infection and highlights the role of the interprofessional team in evaluating and treating patients with this condition.
Objectives:
- Identify the etiology of Salmonella infection.
- Describe the findings associated with Salmonella infection.
- Review the management considerations for patients with Salmonella.
- Explain the importance of collaboration and communication among the interdisciplinary team to enhance the delivery of care and prevention for patients affected by Salmonella.
Introduction
Salmonella is named after D. E. Salmon, an American bacteriologist, who first isolated the bacteria from a pig intestine in 1884.[1] The Salmonella bacteria is a Gram-negative, motile, hydrogen sulfide producing, an acid-labile facultative intracellular microorganism that commonly causes gastroenteritis worldwide and causes cross-infection between humans and animals. Many animals are known carriers for the Salmonella bacterium. One animal carrier is chickens, which eaten while undercooked can cause Salmonella enteritidis leading to inflammatory diarrhea. Salmonella bacteria also cause significant focal infection in patients with immunocompromised conditions. Overall, there are over 2,500 serovars of Salmonella worldwide.
Etiology
Salmonella is a group of gram-negative bacteria that belong to the family Enterobacteriaceae. The current taxonomy splits the Salmonella genus into 2 species (Salmonella enterica and Salmonella bongori) with approval pending for a third species (Salmonella subterranea). S. enterica contains the following 6 subspecies: Salmonella enterica subspecies. Enterica (subspecies I), S. enterica subspecies. salamae (subspecies II), S. enterica subspecies. arizonae (subspecies IIIa), S. enterica subspecies. diarizonae (subspecies IIIb), S. enterica subspecies. houtenae (subspecies IV), and S. enterica subspecies. indica (subspecies VI).[1] Each Salmonella subspecies contains multiple serovars. S. enterica subspecies. enterica holds the most serovars and possesses the serovars most significant to human pathology. Some of the most common serovars include Salmonella serovars enteritidis, typhimurium, newport, and javiana. Serovars typhi and paratyphi lead to typhoidal salmonella causing typhoid fever and paratyphoid fever, which combine to form enteric fever.[2] Typhoidal Salmonella is limited to humans. Other Salmonella enterica serovars lead to nontyphoidal Salmonella (NTS), which can be found in a wide range of vertebrates, including humans, with some serovars restricted to nonhuman vertebrate species.
Epidemiology
In developed countries, like the United States, the most common form of Salmonella infection presents as gastroenteritis and enterocolitis. Over 1 million cases on NTS present annually in the United States. Enteric fever is rare in the United States and is usually acquired from outside the country. However, it is a global concern, with over 20 million new infections annually and greater than 200,000 deaths annually.[3] The incidence of typhoid fever is high in south Asia and sub-Saharan Africa, reaching as high as greater than 100 per 100,000 people per year.[4] The incidence and infection risk in these areas are high, particularly in low to middle-income countries due to poor sanitation along with contaminated food and water sources from infected human feces.
Pathophysiology
The NTS typically causes gastroenteritis, while S. typhi causes systemic disease through the use of virulence factors. One type of virulence factor produced by S. typhi and not NTS includes the Vi antigen, which is a polysaccharide capsule that prevents phagocytosis and destruction from immune cells. Another virulence factor specific to typhoidal Salmonella includes the typhoid toxin. The typhoid toxin is made when the Salmonella bacterium is intracellular and secreted through vesicles to the extracellular space leading to reduced circulating neutrophils, lethargy, and other neurological complications. Other significant virulence factors include the flagella, which helps promote the bacterium’s motility and the type III secretion system, which allows the attachment and insertion process into human nonphagocytic cells.[5] Through the use of these virulence factors, Salmonella enters the intestinal epithelium through the M cells before reaching lymphoid cells of the Peyer patches. After crossing the intestinal epithelium, Salmonella enters macrophages and is disseminated throughout the reticuloendothelial system before causing systemic infection.[6]
History and Physical
In general, Salmonella infection can be involved in bacteremia and focal infections such as osteomyelitis, meningitis, gastroenteritis, and urinary tract infections. Salmonella osteomyelitis is common in sickle cell disease patients. The history and presentation of Salmonella are split into NTS infection and enteric fever. NTS infection most commonly presents as gastroenteritis with diarrhea, fever, and abdominal cramps. Commonly in history, NTS infection is the contact with reptiles, especially in zoos, aquariums, or as pets. For Salmonella enteritidis, eating undercooked chickens may cause inflammatory diarrhea. Other raw or undercooked products such as eggs, meat, and dairy products can also cause Salmonella infection and may be found in the history of the patient. An immunocompromised state is also common for Salmonella infection. Infection is common in children less than one year of age due to underdeveloped immune systems. In adults, Salmonella infection is present in chronic steroid use, malignancy, immunodeficiency from organ transplantation, and acquired immunodeficiency syndrome. S. enterica subspecies. arizonae is a common cause of infection in patients with immunodeficiency states.[7]
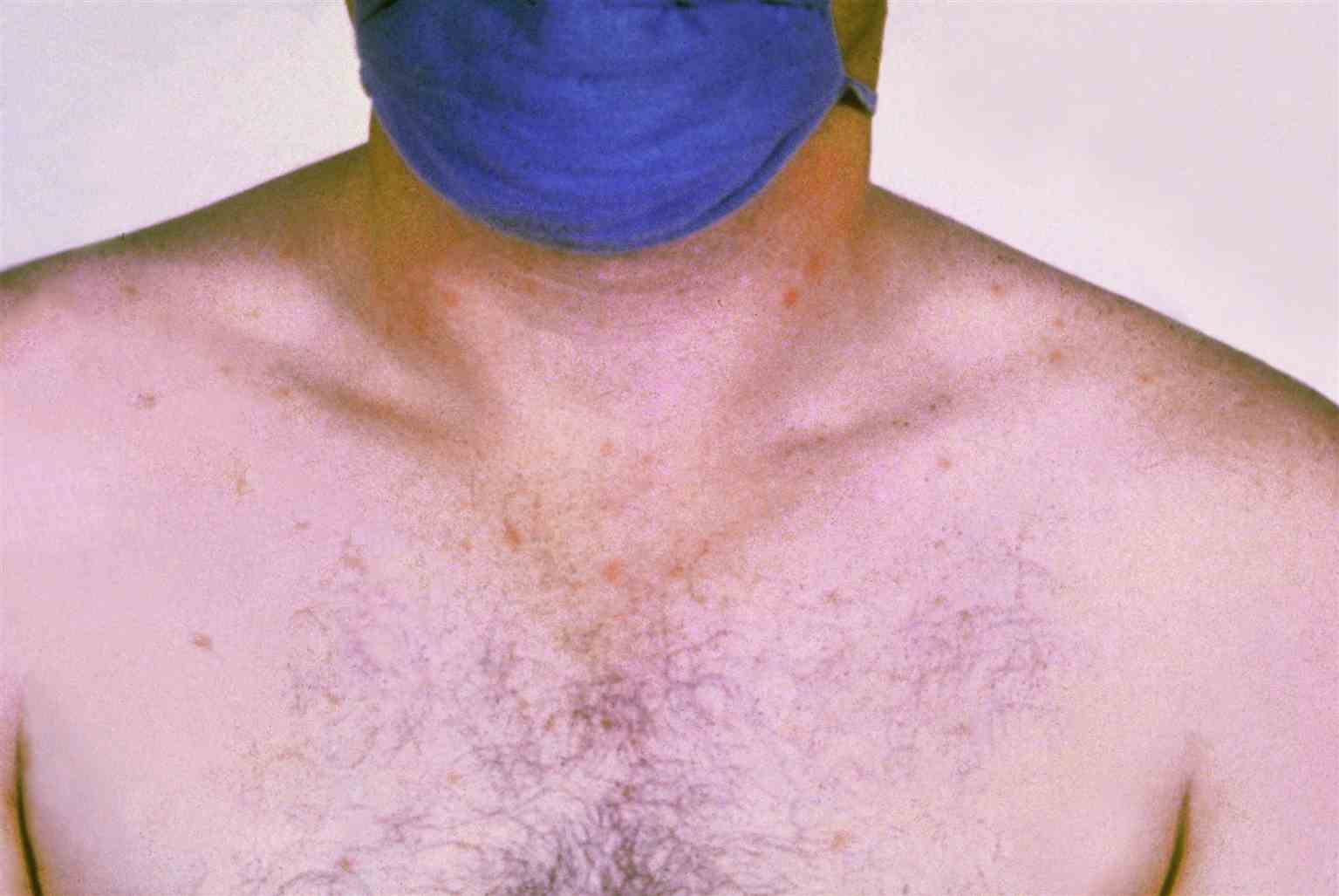
For enteric fever, the onset is gradual and can present with fever and constitutional symptoms such as headache, malaise, and lethargy. Other physical manifestations include abdominal pain, dactylitis, hepatosplenomegaly, and blanching erythematous macular or maculopapular rash famously known as rose spots. Early symptoms include either pea soup diarrhea or constipation. Severe presentations may also further develop into sepsis, shock, and altered mental status from potential meningitis. Bloody diarrhea can occur in a small portion of enteric fever patients.[8] Relative bradycardia compared to elevated temperature states such as fever may also present in enteric fever patients. Enteric fever can develop into a chronic condition leading to patients becoming chronic carriers of the disease. The bacteria are stored in the gallbladder of chronic carriers, which can lead to gallbladder cancer.[9]
Evaluation
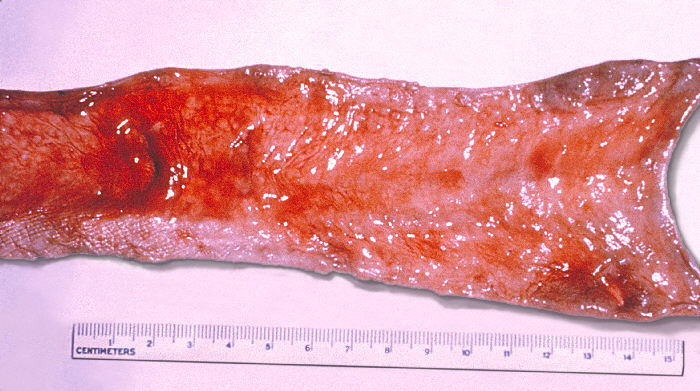
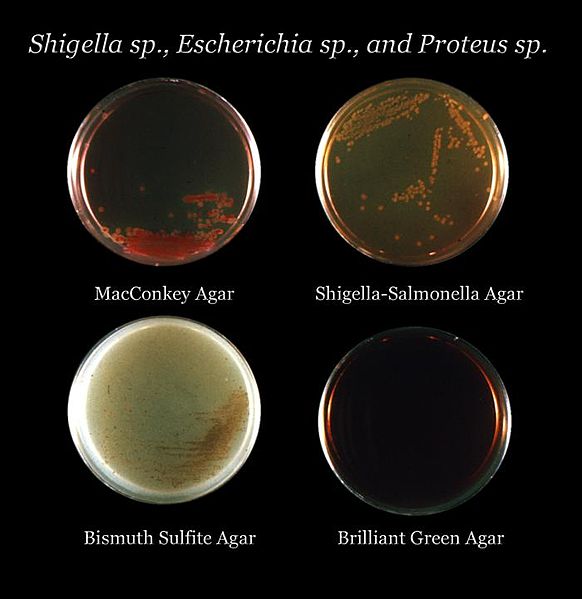
The gold standard for diagnosis of Salmonella infection is by the culture at the focus of infection. The options for culture include stool, blood, urine, bile, cerebrospinal fluid, and bone marrow. Stool culture is used for the diagnosis of Salmonella gastroenteritis and should be considered in severe persistent or bloody diarrhea. Stool culture is primarily for NTS due to high rates of gastroenteritis. For enteric fever, blood, bone marrow, and bile culture are more diagnostic compared to stool culture due to low presence in the stool. Acquisition of cerebrospinal fluid for culture through lumbar puncture is critical for Salmonella meningitis diagnosis. Further identification of Salmonella can occur through growth on selective agar plates such as black colonies on Hektoen Agar plates due to hydrogen sulfide formation. For patients with enteric fever, bone marrow culture is more sensitive than blood culture due to higher concentrations of Salmonella bacteria in the bone marrow compared to the blood.[10] Newer diagnostic modalities include polymerase chain reaction (PCR) tests. PCR is more prominent in research laboratories and is becoming more common in the clinical setting.
Treatment / Management
In most cases, treatment focus should be on the correction of dehydration and electrolyte disturbances. Supportive care is necessary for acute diarrhea and dehydration symptoms. Antipyretic therapy may also be provided if needed. Uncomplicated NTS localized to gastroenteritis without sepsis symptoms is not treated with antibiotics except patients less than 3 months of age and patients with immunocompromised states. Once NTS gastroenteritis is identified, blood cultures should be obtained. In the setting of NTS bacteremia or disseminated disease, initial therapy should be with third-generation cephalosporins like ceftriaxone for at least 7 to 10 days. Once bacterial susceptibilities are known, antibiotic treatment can be transitioned to azithromycin or a fluoroquinolone. Duration of treatment may be extended in certain focal infections such as meningitis (4 weeks) or osteomyelitis (4 to 6 weeks).
For enteric fever, the antibiotic treatment of choice is a fluoroquinolone. However, multidrug-resistant enteric fever is becoming more common globally, leading to a greater focus on susceptibility testing and the use of alternative antibiotics such as third-generation cephalosporins and azithromycin. Fluoroquinolones are not used in children as often compared to adults, and alternatives like azithromycin are often preferred. Treatment duration is typically 10 to 14 days. In cases of severe enteric fever with symptoms of delirium, obtundation, stupor, or shock, additional treatment with corticosteroids may be considered. Dexamethasone at 3 mg/kg, followed by 1 mg/kg every 6 hours for 48 hours, has been shown to decrease mortality. Chronic carriers of the bacteria require four weeks of fluoroquinolone therapy for resolution of carrier status. In adults, if antimicrobial therapy fails, cholecystectomy may be considered due to the persistence of the bacteria in the gallbladder.[10][11]
Differential Diagnosis
Salmonella infections resemble many febrile illnesses. Diagnosis may be confused with alternative conditions in similar regions where Salmonella infection is endemic, such as acute gastroenteritis, viral infections, infection with intracellular microorganisms, and other bacterial infections. Differential diagnosis includes malaria, tuberculosis, brucellosis, tularemia, leptospirosis, encephalitis, endocarditis, influenza, dengue, infectious mononucleosis, and visceral leishmaniasis. Travel history is important for considering typhoid fever due to infection being a common occurrence outside of the United States.[11]
Prognosis
Most individuals with NTS infection recover fully with no further sequelae. However, malnourished patients with poor supportive care may develop prolonged diarrhea, dehydration, and other complications. Individuals requiring antibiotic treatment (infants and patients with immunocompromised states) may have a prolonged disease course with increased disease severity and often have systemic involvement. For enteric fever, patients tend to fully recover if rapidly diagnosed and appropriately treated. If the diagnosis is delayed and appropriate antibiotics and dosages are not used, then adverse outcomes tend to occur. Malnourished patients infected with multidrug-resistant strains tend to have more complications. Chronic carriers occur when patients fail to respond to antibiotic therapy and excrete the bacteria for greater than three months. Chronic carriers are rare in children but tend to increase in age and patients with gallbladder disease. Patients can become chronic urinary carriers when coinfected with schistosomiasis.
Complications
Patients with NTS gastroenteritis may present with dehydration if not properly treated. Complications tend to exist when combined with other diseases. Patients with immunocompromised conditions can present with the systemic infection involving multiple organs leading to sepsis, shock, and death.
Typhoid fever can present with neurological complications such as delirium, psychosis, acute cerebellar ataxia, and Guillain-Barré syndrome. Cardiovascular complications from typhoid fever include myocarditis and pericarditis. Gastrointestinal complications include intestinal hemorrhage and perforation. Enteric fever can lead to chronic carriers if unresponsive to treatment or inappropriately treated.
Deterrence and Patient Education
Prevention of Salmonella infection is focused on several categories, including sanitation, child care with pets, and vaccination. Preventing Salmonella infection through good sanitation practices involves clean water supply and proper food handling practices. Contamination of water supplies can promote Salmonella infection, and chlorination with the purification of water is essential in preventing infection. Food must be processed properly in kitchens. Food handlers must practice good hand washing protocols. Street food like fruit and ice cream along with animal products like raw eggs, chicken, and uncooked meat are important sources of infection.
Pets and child care is an important source of Salmonella infection. Reptiles and amphibians are a known source of Salmonella transmission to humans. After handling these animals, individuals should wash their hands. There should be no food or drink in areas where animal handling occurs. These pets should not be found in child care centers or should be inaccessible to children less than five years of age. Patients with immunocompromised states should not be handling reptiles and amphibians. Children infected with Salmonella may return to school and daycare centers once symptoms like diarrhea have resolved. In Salmonella typhi or paratyphi 3, negative stool cultures have to be documented before the child is allowed to return to school.
Vaccination is a current method to prevent typhoid fever. Two vaccines provide protection against Salmonella serovar typhi. They are recommended to travelers, people with intimate exposure to typhoid fever carriers, and laboratory workers with exposure to Salmonella. The first vaccine, Ty21a strain typhoid vaccine, is a live attenuated oral vaccine given to patients six years and older. The vaccine is administered in 4 doses on days 0, 2, 4, and 6. Vaccination should be completed one week before exposure. Boosters, with all 4 oral doses, should be completed every 5 years. Antimicrobials like proguanil, mefloquine, chloroquine, and other antibiotics should not be given at least 3 days before starting vaccination and at least 3 days after completing vaccination depending on the pharmaceutical. The second vaccine is a Vi capsular polysaccharide vaccine (ViCPS) given to patients 2 years and older. It is given as a one-time intramuscular injection at least 2 weeks before exposure. Boosters are given every 2 years.[12][13]
Enhancing Healthcare Team Outcomes
Salmonella infection can be difficult to diagnose at first due to nonspecific symptoms. Diagnosis is usually confirmed by culture, followed by treatment with supportive care and/or antibiotics. Usually, treatment is completed by an inpatient team, which consists of at least the physician and nurse. The nurse and physician take care of and manage the patient while hospitalized. The laboratory and personnel are also involved with blood work, follow up of culture results, and antibiotic susceptibility during the hospitalization. The pharmacist assists in medication decisions and dosage for the patient. In addition to the primary hospital team, an infectious disease physician may be consulted to assist in treatment, management, and follow-up of the patient after the hospital admission. This physician may also coordinate with public health services to prevent Salmonella spread in the community. Public health services and prevention may be the next step to lower Salmonella infection rates. Many groups have worked on vaccines to prevent infection. Besides the two known vaccines, a Vi tetanus toxoid conjugate vaccine has been studied and has shown promise in preventing infection in randomized control trials.[14] [Level 1]