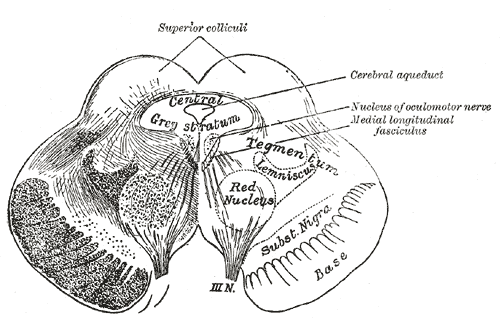
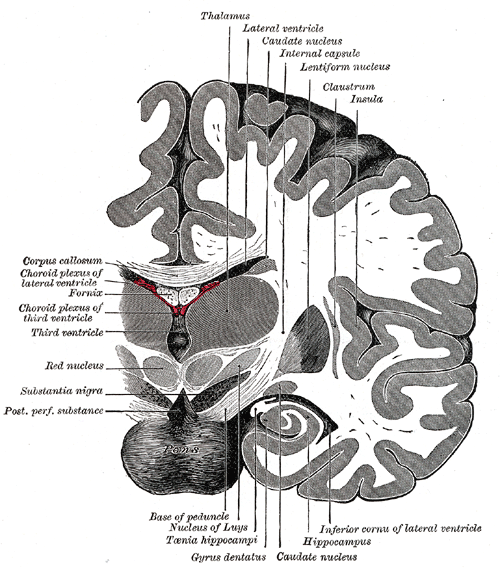
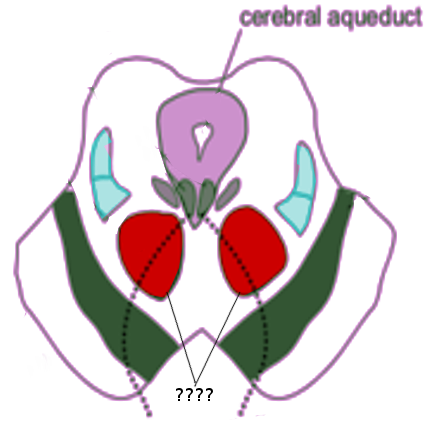
[1]
Cacciola A, Milardi D, Basile GA, Bertino S, Calamuneri A, Chillemi G, Paladina G, Impellizzeri F, Trimarchi F, Anastasi G, Bramanti A, Rizzo G. The cortico-rubral and cerebello-rubral pathways are topographically organized within the human red nucleus. Scientific reports. 2019 Aug 20:9(1):12117. doi: 10.1038/s41598-019-48164-7. Epub 2019 Aug 20
[PubMed PMID: 31431648]
[2]
Yamaguchi K, Goto N. Development of the human magnocellular red nucleus: a morphological study. Brain & development. 2006 Aug:28(7):431-5
[PubMed PMID: 16516425]
[4]
ten Donkelaar HJ. Evolution of the red nucleus and rubrospinal tract. Behavioural brain research. 1988 Apr-May:28(1-2):9-20
[PubMed PMID: 3289562]
[5]
Onodera S, Hicks TP. A comparative neuroanatomical study of the red nucleus of the cat, macaque and human. PloS one. 2009 Aug 13:4(8):e6623. doi: 10.1371/journal.pone.0006623. Epub 2009 Aug 13
[PubMed PMID: 19675676]
Level 2 (mid-level) evidence
[6]
Patt S, Gerhard L, Zill E. A Golgi study on the red nucleus in man. Histology and histopathology. 1994 Jan:9(1):7-10
[PubMed PMID: 8003824]
[7]
Miller LE, van Kan PL, Sinkjaer T, Andersen T, Harris GD, Houk JC. Correlation of primate red nucleus discharge with muscle activity during free-form arm movements. The Journal of physiology. 1993 Sep:469():213-43
[PubMed PMID: 8271199]
[8]
Lavoie S, Drew T. Discharge characteristics of neurons in the red nucleus during voluntary gait modifications: a comparison with the motor cortex. Journal of neurophysiology. 2002 Oct:88(4):1791-814
[PubMed PMID: 12364507]
[9]
Ulfig N, Chan WY. Differential expression of calcium-binding proteins in the red nucleus of the developing and adult human brain. Anatomy and embryology. 2001 Feb:203(2):95-108
[PubMed PMID: 11218063]
[10]
Hicks TP, Onodera S. The mammalian red nucleus and its role in motor systems, including the emergence of bipedalism and language. Progress in neurobiology. 2012 Feb:96(2):165-75. doi: 10.1016/j.pneurobio.2011.12.002. Epub 2012 Jan 2
[PubMed PMID: 22230734]
[11]
Yang JF, Stephens MJ, Vishram R. Infant stepping: a method to study the sensory control of human walking. The Journal of physiology. 1998 Mar 15:507 ( Pt 3)(Pt 3):927-37
[PubMed PMID: 9508851]
[12]
Reid EK, Norris SA, Taylor JA, Hathaway EN, Smith AJ, Yttri EA, Thach WT. Is the parvocellular red nucleus involved in cerebellar motor learning? Current trends in neurology. 2009 Jan 1:3():15-22
[PubMed PMID: 21743781]
[13]
Lang EJ, Apps R, Bengtsson F, Cerminara NL, De Zeeuw CI, Ebner TJ, Heck DH, Jaeger D, Jörntell H, Kawato M, Otis TS, Ozyildirim O, Popa LS, Reeves AM, Schweighofer N, Sugihara I, Xiao J. The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper. Cerebellum (London, England). 2017 Feb:16(1):230-252. doi: 10.1007/s12311-016-0787-8. Epub
[PubMed PMID: 27193702]
Level 3 (low-level) evidence
[14]
Fisher CM. The posterior cerebral artery syndrome. The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 1986 Aug:13(3):232-9
[PubMed PMID: 3742339]
[16]
Huang M, Liu M, Li X. [The analgesic effect of red nucleus and preliminary research on its mechanism]. Zhen ci yan jiu = Acupuncture research. 1992:17(3):166-70
[PubMed PMID: 1339625]
[17]
Steffens H, Rathelot JA, Padel Y. Effects of noxious skin heating on spontaneous cell activity in the magnocellular red nucleus of the cat. Experimental brain research. 2000 Mar:131(2):215-24
[PubMed PMID: 10766273]
[18]
Guo YJ, Li HN, Ding CP, Han SP, Wang JY. Red nucleus interleukin-1β evokes tactile allodynia through activation of JAK/STAT3 and JNK signaling pathways. Journal of neuroscience research. 2018 Dec:96(12):1847-1861. doi: 10.1002/jnr.24324. Epub 2018 Sep 14
[PubMed PMID: 30216497]
[19]
Ding CP, Guo YJ, Li HN, Wang JY, Zeng XY. Red nucleus interleukin-6 participates in the maintenance of neuropathic pain through JAK/STAT3 and ERK signaling pathways. Experimental neurology. 2018 Feb:300():212-221. doi: 10.1016/j.expneurol.2017.11.012. Epub 2017 Nov 26
[PubMed PMID: 29183675]
[20]
Zhang Q, Wang J, Duan MT, Han SP, Zeng XY, Wang JY. NF-κB, ERK, p38 MAPK and JNK contribute to the initiation and/or maintenance of mechanical allodynia induced by tumor necrosis factor-alpha in the red nucleus. Brain research bulletin. 2013 Oct:99():132-9. doi: 10.1016/j.brainresbull.2013.10.008. Epub 2013 Oct 23
[PubMed PMID: 24161765]
[21]
Wang J, Yu J, Ding CP, Han SP, Zeng XY, Wang JY. Transforming growth factor-beta in the red nucleus plays antinociceptive effect under physiological and pathological pain conditions. Neuroscience. 2015 Apr 16:291():37-45. doi: 10.1016/j.neuroscience.2015.01.059. Epub 2015 Feb 4
[PubMed PMID: 25662509]
[22]
Wang ZH, Zeng XY, Han SP, Fan GX, Wang JY. Interleukin-10 of red nucleus plays anti-allodynia effect in neuropathic pain rats with spared nerve injury. Neurochemical research. 2012 Aug:37(8):1811-9. doi: 10.1007/s11064-012-0795-0. Epub 2012 May 15
[PubMed PMID: 22584848]
[23]
Ding CP, Xue YS, Yu J, Guo YJ, Zeng XY, Wang JY. The Red Nucleus Interleukin-6 Participates in the Maintenance of Neuropathic Pain Induced by Spared Nerve Injury. Neurochemical research. 2016 Nov:41(11):3042-3051
[PubMed PMID: 27485712]
[24]
Li X, Wang J, Wang Z, Dong C, Dong X, Jing Y, Yuan Y, Fan G. Tumor necrosis factor-α of Red nucleus involved in the development of neuropathic allodynia. Brain research bulletin. 2008 Nov 25:77(5):233-6. doi: 10.1016/j.brainresbull.2008.08.025. Epub 2008 Sep 25
[PubMed PMID: 18824078]
[25]
Zhang Q, Yu J, Wang J, Ding CP, Han SP, Zeng XY, Wang JY. The Red Nucleus TNF-α Participates in the Initiation and Maintenance of Neuropathic Pain Through Different Signaling Pathways. Neurochemical research. 2015 Jul:40(7):1360-71. doi: 10.1007/s11064-015-1599-9. Epub 2015 May 8
[PubMed PMID: 25952358]
[26]
Okun MS, Tagliati M, Pourfar M, Fernandez HH, Rodriguez RL, Alterman RL, Foote KD. Management of referred deep brain stimulation failures: a retrospective analysis from 2 movement disorders centers. Archives of neurology. 2005 Aug:62(8):1250-5
[PubMed PMID: 15956104]
Level 2 (mid-level) evidence
[27]
Rodriguez-Oroz MC, Rodriguez M, Leiva C, Rodriguez-Palmero M, Nieto J, Garcia-Garcia D, Luis Zubieta J, Cardiel C, Obeso JA. Neuronal activity of the red nucleus in Parkinson's disease. Movement disorders : official journal of the Movement Disorder Society. 2008 Apr 30:23(6):908-11. doi: 10.1002/mds.22000. Epub
[PubMed PMID: 18383534]
[28]
Merola A, Mandybur G, Biddell K, Tareen TK, Wilson-Perez H, Espay AJ, Duker AP. Subthalamic or red nucleus? A puzzling question arising during intraoperative recording for DBS. Clinical neurophysiology : official journal of the International Federation of Clinical Neurophysiology. 2017 Apr:128(4):558-560. doi: 10.1016/j.clinph.2017.01.011. Epub 2017 Jan 28
[PubMed PMID: 28231473]
[29]
Ellis TM, Foote KD, Fernandez HH, Sudhyadhom A, Rodriguez RL, Zeilman P, Jacobson CE 4th, Okun MS. Reoperation for suboptimal outcomes after deep brain stimulation surgery. Neurosurgery. 2008 Oct:63(4):754-60; discussion 760-1. doi: 10.1227/01.NEU.0000325492.58799.35. Epub
[PubMed PMID: 18981887]
[30]
Kim H, Lee H, Jung KI, Ohn SH, Yoo WK. Changes in Diffusion Metrics of the Red Nucleus in Chronic Stroke Patients With Severe Corticospinal Tract Injury: A Preliminary Study. Annals of rehabilitation medicine. 2018 Jun 27:42(3):396-405. doi: 10.5535/arm.2018.42.3.396. Epub 2018 Jun 27
[PubMed PMID: 29961737]
[31]
Yang HS, Kwon HG, Hong JH, Hong CP, Jang SH. The rubrospinal tract in the human brain: diffusion tensor imaging study. Neuroscience letters. 2011 Oct 17:504(1):45-8. doi: 10.1016/j.neulet.2011.08.054. Epub 2011 Sep 3
[PubMed PMID: 21911039]
Level 3 (low-level) evidence
[32]
Yeo SS, Jang SH. Changes in red nucleus after pyramidal tract injury in patients with cerebral infarct. NeuroRehabilitation. 2010:27(4):373-7. doi: 10.3233/NRE-2010-0622. Epub
[PubMed PMID: 21160128]
[33]
Belhaj-Saïf A, Cheney PD. Plasticity in the distribution of the red nucleus output to forearm muscles after unilateral lesions of the pyramidal tract. Journal of neurophysiology. 2000 May:83(5):3147-53
[PubMed PMID: 10805709]
[34]
Rüber T, Schlaug G, Lindenberg R. Compensatory role of the cortico-rubro-spinal tract in motor recovery after stroke. Neurology. 2012 Aug 7:79(6):515-22. doi: 10.1212/WNL.0b013e31826356e8. Epub 2012 Jul 25
[PubMed PMID: 22843266]
[36]
Telford R, Vattoth S. MR anatomy of deep brain nuclei with special reference to specific diseases and deep brain stimulation localization. The neuroradiology journal. 2014 Feb:27(1):29-43
[PubMed PMID: 24571832]
[37]
Pearce JM. Palatal Myoclonus (syn. Palatal Tremor). European neurology. 2008:60(6):312-5. doi: 10.1159/000159929. Epub 2008 Oct 3
[PubMed PMID: 18832845]
[38]
Brazis PW. Localization of lesions of the oculomotor nerve: recent concepts. Mayo Clinic proceedings. 1991 Oct:66(10):1029-35
[PubMed PMID: 1921485]
[39]
Bandt SK, Anderson D, Biller J. Deep brain stimulation as an effective treatment option for post-midbrain infarction-related tremor as it presents with Benedikt syndrome. Journal of neurosurgery. 2008 Oct:109(4):635-9. doi: 10.3171/JNS/2008/109/10/0635. Epub
[PubMed PMID: 18826349]
[40]
Akdal G, Kutluk K, Men S, Yaka E. Benedikt and "plus-minus lid" syndromes arising from posterior cerebral artery branch occlusion. Journal of the neurological sciences. 2005 Jan 15:228(1):105-7
[PubMed PMID: 15607218]
[41]
Broadley SA, Taylor J, Waddy HM, Thompson PD. The clinical and MRI correlate of ischaemia in the ventromedial midbrain: Claude's syndrome. Journal of neurology. 2001 Dec:248(12):1087-9
[PubMed PMID: 12013587]
[42]
Louro P, Durães J, Oliveira D, Paiva S, Ramos L, Macário MC. Woodhouse-Sakati Syndrome: First report of a Portuguese case. American journal of medical genetics. Part A. 2019 Nov:179(11):2237-2240. doi: 10.1002/ajmg.a.61303. Epub 2019 Jul 26
[PubMed PMID: 31347785]
Level 3 (low-level) evidence
[43]
Abusrair AH, Bohlega S, Al-Semari A, Al-Ajlan FS, Al-Ahmadi K, Mohamed B, AlDakheel A. Brain MR Imaging Findings in Woodhouse-Sakati Syndrome. AJNR. American journal of neuroradiology. 2018 Dec:39(12):2256-2262. doi: 10.3174/ajnr.A5879. Epub 2018 Nov 8
[PubMed PMID: 30409855]
[44]
Lewis MM, Du G, Kidacki M, Patel N, Shaffer ML, Mailman RB, Huang X. Higher iron in the red nucleus marks Parkinson's dyskinesia. Neurobiology of aging. 2013 May:34(5):1497-503. doi: 10.1016/j.neurobiolaging.2012.10.025. Epub 2012 Nov 21
[PubMed PMID: 23177595]
[45]
Philippens IHCHM, Wubben JA, Franke SK, Hofman S, Langermans JAM. Involvement of the Red Nucleus in the Compensation of Parkinsonism may Explain why Primates can develop Stable Parkinson's Disease. Scientific reports. 2019 Jan 29:9(1):880. doi: 10.1038/s41598-018-37381-1. Epub 2019 Jan 29
[PubMed PMID: 30696912]
[46]
Camlidag I, Kocabicak E, Sahin B, Jahanshahi A, Incesu L, Aygun D, Yildiz O, Temel Y, Belet U. Volumetric analysis of the subthalamic and red nuclei based on magnetic resonance imaging in patients with Parkinson's disease. The International journal of neuroscience. 2014 Apr:124(4):291-5. doi: 10.3109/00207454.2013.843091. Epub 2013 Sep 26
[PubMed PMID: 24020352]
[47]
Louis ED, Lenka A. The Olivary Hypothesis of Essential Tremor: Time to Lay this Model to Rest? Tremor and other hyperkinetic movements (New York, N.Y.). 2017:7():473. doi: 10.7916/D8FF40RX. Epub 2017 Jul 13
[PubMed PMID: 28966877]