Introduction
The human larynx is a specialized organ complexly organized of multiple tissue types. Although the intricate care, study, and surgical management of the larynx fall under the specialty of otolaryngology, knowledge of the larynx and associated conditions is beneficial for many medical specialties. Its position in the human body and unique functions allow the pathophysiology of the larynx to involve a spectrum of conditions and treatments from lifesaving respiratory interventions to improvements in voice quality.
Structure and Function
The larynx is a cartilaginous skeleton with intricate neuromuscular control. The hyoid is the only bone in the larynx and articulates with many of the extrinsic muscles of the larynx. A notable external landmark of the larynx is the anterior prominence of the thyroid cartilage. The hyoid bone is palpable superiorly, and the thyroid gland is palpable inferiorly.
The structure of the larynx divides into three basic parts: the supraglottis, the glottis, and the subglottis.[1]
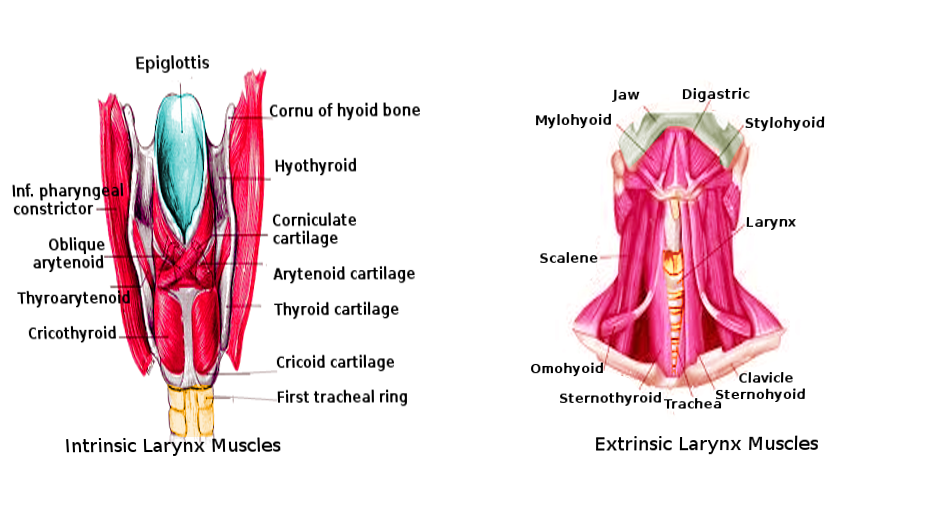
The cartilages of the supraglottis are the epiglottis, two arytenoids, and two corniculate cartilages. The arytenoid cartilages contain a vocal process medially from which the vocal ligament extends anteriorly to the thyroid cartilage. The vestibular folds (false cords) are folds of mucosa positioned superolateral to the vocal ligaments. The lateral surface of the arytenoid contains the muscular process to which several intrinsic laryngeal muscles attach[1]. The aryepiglottic folds connect the superior surface of the arytenoids to the lateral border of the epiglottis. The inferior aspect of the arytenoids articulate with the cricoid cartilage. The corniculate cartilages are positioned superiorly to the arytenoids without muscular insertion. Finally, the cuneiform cartilages exist as sesamoid cartilages within the aryepiglottic folds.
The anatomy of the glottis is understood to be divided into the phonatory (anterior glottis) and respiratory (posterior glottis) portions functionally.[2] The boundaries of the glottis are relative to the structures of the supraglottis and subglottis. Its superior border is the middle of the ventricle, and its inferior border lies 1 cm beneath the vocal folds.
The inferior-most portion of the larynx is the subglottis. It extends from 1 cm below the vocal ligaments to the inferior border of the cricoid cartilage.[2] The major cartilaginous structures of the subglottis are the cricoid cartilage and the thyroid cartilage. The cricoid cartilage is the only cartilaginous structure of the larynx to surround the airway. Extending superiorly from the cricoid cartilage is the conus elasticus. At its superior most border, it thickens to form the vocal ligaments of the glottis.[3]
The primary functions of the larynx are in respiration, airway protection during deglutition, and phonation. In respiration, vocal folds are optimally in abduction, increasing the total area of the glottis to facilitate work of breathing.[4] When swallowing, the epiglottis is pulled posteroinferiorly to cover the glottis, allowing the bolus to pass safely into the hypopharynx and esophagus without compromising the airway. In phonation, the vocal ligaments, muscles and overlying mucosa are tensed and adducted to vibrate at frequencies appropriate for human speech.[4]
Embryology
The development of the larynx begins on approximately the third week of gestation with the generation of the laryngotracheal groove in the 6th pharyngeal arch.[5] This groove eventually deepens to form the laryngotracheal diverticulum, also known as the lung bud. The diverticulum extends rostrally to develop the trachea and rest of the lower respiratory system. The mucosa of the larynx derives from the endoderm of the pharyngeal arches, whereas the cartilage and musculature of the larynx arise from the mesoderm.[5] The majority of laryngeal structures form from the fourth (thyroid cartilage, cuneiform cartilages, part of the epiglottis, superior laryngeal nerve, cricopharyngeus muscles, and cricothyroid muscles) and sixth(recurrent laryngeal nerves, cricoid cartilages, arytenoid cartilages, corniculate cartilages, and remaining intrinsic muscles). The second pharyngeal arch contributes to the majority of the hyoid bone, and the third pharyngeal arch develops the greater cornu of the hyoid and a portion of the epiglottis.
By ten weeks of gestation, the laryngeal inlet develops a covering membrane.[5] This membrane recanalizes to opening the larynx to the lower respiratory system and forming the vestibule of the larynx.
Blood Supply and Lymphatics
The blood supply to the originates from branches off of the thyroid arteries. The superior thyroid artery branches from the external carotid artery inferior to the greater cornu of the hyoid bone and gives rise to two arteries of the larynx: the superior laryngeal artery and the cricothyroid artery.[1] The superior laryngeal artery follows the course of the internal branch of the superior laryngeal nerve and pierces the thyrohyoid membrane to give blood supply to the superior structures of the larynx. The cricothyroid artery predominately supplies the cricothyroid muscle. The blood supply to the inferior larynx originates from the thyrocervical trunk with the inferior thyroid artery. This vessel gives rise to the inferior laryngeal artery anterior to the inferior pharyngeal constrictor muscle and posterior to the trachea. Its course follows the course of the recurrent laryngeal nerve. The venous drainage of the larynx follows a similar pattern to its supply.[1]
Lymphatic drainage is important to understand due to the aggressive metastasis of some laryngeal cancers. Though the lymphatic drainage can vary individually, there are understood consistencies. Supraglottic structures drain lymph to the deep cervical lymph nodes, which can further subdivide into levels. Subglottic structures drain lymph to the paratracheal and pretracheal lymph nodes.[1]
Nerves
The major contributor of innervation to the larynx is the vagus nerve, cranial nerve X, which innervates the intrinsic laryngeal musculature. The extrinsic musculature innervation divides between the vagus nerve and the cervical rootlets.
The laryngeal branches of the vagus nerve are the superior laryngeal nerve and the recurrent laryngeal nerve. The superior laryngeal nerve has two main branches - the external branch and the internal branch. The external branch of the superior laryngeal nerve carries somatic motor fibers to the cricothyroid muscle as its sole function.[6] The internal branch enters the larynx through a foramen in the thyrohyoid membrane with the superior thyroid artery providing somatosensory innervation to the mucosa of the glottis and supraglottis. The recurrent laryngeal nerve branches inferior to the superior laryngeal nerve and supplies motor sensation to the remaining intrinsic muscles of the larynx. Also, the nerve carries somatosensory fibers to the subglottis and upper trachea.[7] Due to embryologic differences in anatomy, the course of the left recurrent laryngeal nerve differs from its right-sided counterpart. While the right recurrent laryngeal nerve descends anterior to the subclavian artery and then ascends posterior towards the larynx, the left recurrent laryngeal nerve courses around the aorta posterior to the ligamentum arteriosum of the heart.[1]
The cervical rootlets comprise the plexus known as the ansa cervicalis. The ansa provides innervation to many of the extrinsic muscles of the larynx such as the infrahyoid and suprahyoid musculature. The pharyngeal branch of the vagus nerve innervates the pharyngeal constrictors, which act with the suprahyoid muscles to elevate the larynx during swallowing.
Muscles
The intrinsic muscles of the larynx serve a variety of functions, many of which can be categorized by their action on the vocal ligament. The adductors are the thyroarytenoid (vocalis), lateral cricoarytenoid, and interarytenoid muscles.[8] The thyroarytenoid originates at the oblique line of the thyroid cartilage and inserts at the anterior surface of the ipsilateral arytenoid cartilage. Its functions include both adducting the cords and decreasing the tension of the vocal ligament to modulate pitch.[7] The lateral cricoarytenoid, which originates on the lateral cricoid cartilage and inserts at the muscular process of the arytenoid, adducts the cords and internally rotates the arytenoid. The interarytenoid (transverse arytenoid) muscle works synergistically with the lateral cricoarytenoid by maintaining the closure of the glottis after the cords have adducted. It originates at the medial aspect of the arytenoid cartilages and inserts into the contralateral interarytenoid muscle.[8] The sole abductor of the vocal ligament is the posterior cricoarytenoid muscle. This muscle originates at the posterior cricoid and inserts on the posterior muscular process of the arytenoid. Its action is to abduct and externally rotate the arytenoid in antagonism to the lateral cricoarytenoid muscle. The cricothyroid muscle originates at the anterolateral surface of the cricoid and inserts on the inferolateral surface of the thyroid cartilage. In contracting, it displaces the thyroid cartilage downward and tenses the vocal cords. It is the only intrinsic muscle of the larynx to receive its innervation from the external branch of the superior laryngeal nerve instead of the recurrent laryngeal nerve.[8]
The epiglottis is drawn posteriorly over the laryngeal inlet to prevent bolus aspiration. The aryepiglottic muscles originate at the apex of the arytenoids and insert at the lateral border of the epiglottis. Similarly, the oblique arytenoid muscles originate at the arytenoid apex and insert at the aryepiglottic fold. The thyroepiglottic muscle originates on the internal surface of the thyroid cartilage and inserts on the margin of the epiglottis. When contracting, this muscle inferiorly displaces the epiglottis. These muscles work synergistically to close the laryngeal inlet by pulling the epiglottis posteriorly and inferiorly.
Several cervical muscles act extrinsically on the larynx to aid in phonation and swallowing. Muscles inserted on the superior aspect of the hyoid (geniohyoid, digastric, mylohyoid, thyrohyoid, and stylohyoid muscles) and pharynx (stylopharyngeus, palatopharyngeus, and pharyngeal constrictor muscles) act in conjunction to elevate the larynx. Those muscles inserted on the inferior surface of the hyoid (sternohyoid and omohyoid muscles) and the sternothyroid muscle act to depress the larynx.[8] Because of the elevation of the larynx during swallowing, physical exam maneuvers to examine the thyroid often involve the patient voluntarily swallowing to localize this gland better.
Physiologic Variants
Small variations in laryngeal musculature can affect voice quality and tone. The differences in voice quality are apparent between male and female larynxes. In males, testosterone thickens the vocal ligaments, causing deeper voice quality. The strength and fine control of the muscles is acquirable from use. Singers can gain improved fine motor control over their intrinsic muscles through years of practice to better modulate pitch. Though anatomic variations can exist in the musculature, cartilaginous variations are more frequent with the thyroid cartilage most commonly affected.[9]
While the innervation of laryngeal muscles frequently appears as described above, variations in innervation are common in the larynx. Often, the superior laryngeal nerve and recurrent laryngeal nerve branches will form a plexus, and aberrant branches will provide motor innervation to nearby muscles.[10]
Surgical Considerations
Hoarseness is a frequent complaint following long surgeries or prolonged admissions to the ICU. Often, the patient’s hoarseness is related to trauma caused by the intubation. Risk factors for intubation-related laryngeal injury are prolonged intubation (greater than 36 hours), intubation without myorelaxant drugs, large endotracheal tubes, aspiration, and coexisting presence of a nasogastric tube.[11] Occasionally, vocal cord paralysis can occur due to the prolonged compression of the recurrent laryngeal nerve between the endotracheal tube balloon and the thyroid cartilage in the subglottis.
Because of its long course through the neck and mediastinum, the recurrent laryngeal nerve is subject to injury in cervical surgery (thyroid surgery, neck dissections, anterior cervical approach to the spine, etc.) and thoracic surgery (lung biopsy, aortic aneurysm repair, etc.). Surgical injury is the leading cause of unilateral vocal cord paralysis, followed by idiopathic and malignant causes.[12] Patients commonly present postoperatively with hoarseness that does not resolve over time.
Clinical Significance
Assessing laryngeal function and mobility is valuable for both the otolaryngologist and primary care physician. As an outpatient, primary care physicians can use laryngeal mirrors to visualize the larynx for basic screening purposes to investigate foreign bodies, masses, or inflammation. To better assess laryngeal function and visualize anatomy more intimately, an otolaryngologist or another trained practitioner can use a flexible laryngoscope. After the nasal administration of a topical anesthetic, the scope is passed through the nasopharynx as far as tolerated by the patient. Performing this well-tolerated procedure on an alert patient allows for outpatient visualization of the dynamic larynx. For most diagnostic visualization and biopsies, rigid laryngoscopy under sedation is necessary.[2]
Hoarseness and dysphagia can be the presenting symptoms of countless diseases. Trauma, stroke, malignancy, neuromuscular disorders, and psychiatric illness can cause combinations of these diseases. An appropriate differential can be generated based on demographics, exposures, and detailed history.