Continuing Education Activity
While uncommon, acute aortic dissection is an infrequent but catastrophic disorder. Patients present with tearing chest pain that radiates to the back. It is essential to differentiate aortic dissection from other causes of chest pain. This activity describes the etiology, classification, pathophysiology, evaluation, and management of aortic dissection and highlights the role of interprofessional teams in improving outcomes for such patients.
Objectives:
- Describe the etiology of patients with aortic dissection.
- Outline the evaluation in patients with aortic dissection.
- Review the conservative and surgical treatment options available for patients with aortic dissection.
- Explain interprofessional team strategies for enhancing care coordination to facilitate rapid diagnosis and targeted management of patients with aortic dissection.
Introduction
While uncommon, acute aortic dissection (AAD) is a rare but catastrophic disorder. Aortic dissection is due to the separation of the layers of the aortic wall. A tear in the intimal layer results in the progression of the dissection (either proximal or retrograde) chiefly due to the entry of blood in between the intima and media. An acute aortic dissection is associated with very high mortality; the majority die even before reaching the emergency department. Patients with a chronic aortic dissection (more than two weeks) have a slightly better prognosis.
Classically described as a patient complaining of an abrupt onset of severe ‘tearing’ chest pain, presentations can often be more subtle. Physicians correctly suspect the diagnosis in as few as 15% to 43% of cases of verified AAD. If left untreated, mortality approaches 50% in the first 48 hours of onset. Despite a wealth of literature, a significant number of aortic dissections are missed in the emergency department.[1][2][3]
There are two main anatomic classifications used to classify aortic dissection.
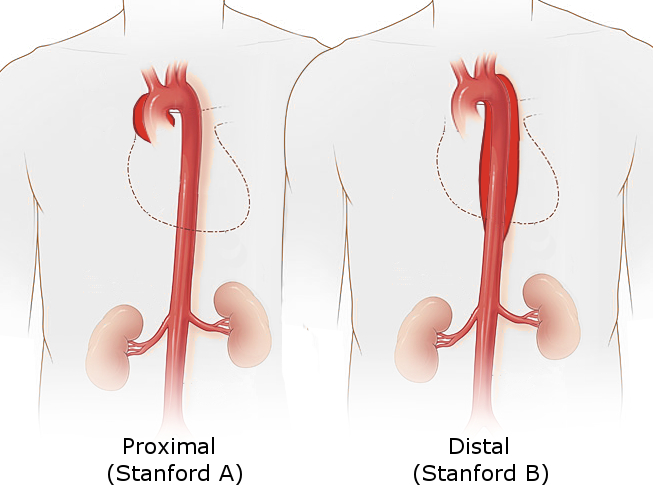
The Stanford system is more frequently employed. It classifies dissections into two types based on whether ascending or descending part of the aorta involved.
- Type A involves the ascending aorta, regardless of the site of the primary intimal tear. Type A dissection is defined as a dissection proximal to the brachiocephalic artery.
- Type B aortic dissection originating distal to the left subclavian artery and involving only descending aorta.
The DeBakey classification is based upon the site of origin of the dissection.
- Type 1 originates in the ascending aorta and to at least the aortic arch.
- Type 2 originates in and is limited to the ascending aorta.
- Type 3 begins in the descending aorta and extends distally above the diaphragm (type 3a) or below the diaphragm (type 3b).
Ascending aortic dissections are almost twice as common as descending dissections.[4]
Etiology
Predisposing high-risk factors for non-traumatic aortic dissection include:
- Hypertension (occurs in 70% of patients with distal Standford type B AAD)
- An abrupt, transient, severe increase in blood pressure (e.g., strenuous weight lifting and use of sympathomimetic agents such as cocaine, ecstasy, or energy drinks)
- Genetic conditions including Marfan syndrome (In an IRAD review, Marfan syndrome was present in 50% of those under age 40, compared with only 2% of older patients), Ehlers-Danlos syndrome, Turner syndrome, and bicuspid aortic valve, coarctation of the aorta. In patients with Marfan syndrome, cystic medial necrosis is seen in the tissues
- Pre-existing aortic aneurysm
- Atherosclerosis
- Pregnancy and delivery (risk compounded in pregnant women with connective tissue disorders such as Marfan syndrome)
- Family history
- Aortic instrumentation or surgery (coronary artery bypass, aortic or mitral valve replacement, and percutaneous stenting or catheter insertion)
- Inflammatory or infectious diseases that cause vasculitis (syphilis, cocaine use)
Epidemiology
The incidence of aortic dissection is reported to be 5 to 30 cases per 1 million people per year (compared to the much more common condition of acute myocardial infarction, which affects approximately 4400 cases per 1,000,000 person-years). Regarding emergency department presentations, three AADs are ultimately diagnosed out of every 1000 patients presenting with acute back, chest, or abdominal pain. Age is a risk factor for approximately 75% of aortic dissections occurring in patients who are ages 40 to 70 years, with the majority occurring between the ages of 50 and 65 years. However, there are some significant differences between older adult patients and younger patients with dissections involving the ascending aorta. Older patients are significantly more likely to harbor atherosclerosis, prior aortic aneurysm, iatrogenic dissection, or an intramural hematoma. Younger patients are much less likely to have a history of hypertension and much more likely to suffer from a connective tissue disorder such as Marfan syndrome. Additionally, AAD is three times more common in men than in women, although women tend to present later and experience worse outcomes.[5]
Pathophysiology
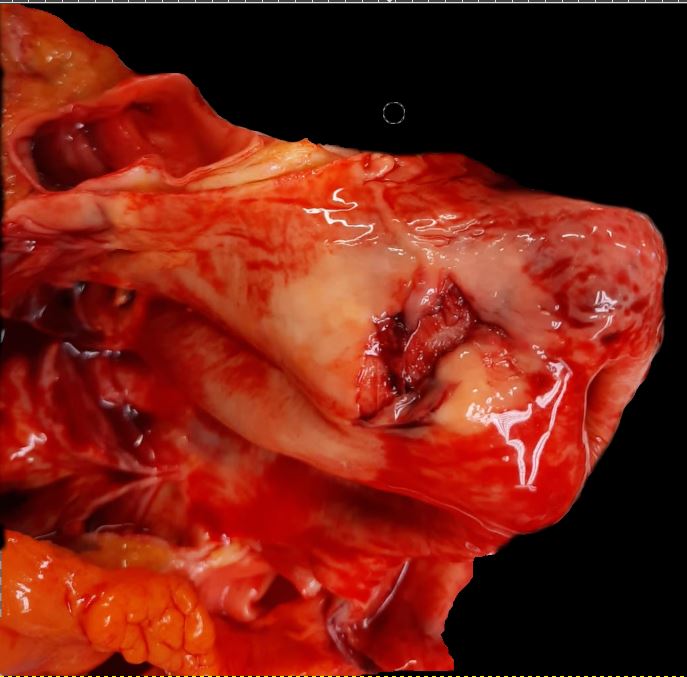
The aortic wall consists of three layers: the intima, media, and adventitia. Constant exposure to high pulsatile pressure and shear stress leads to a weakening of the aortic wall in susceptible patients resulting in an intimal tear. Following this rent, blood flows into the intima-media space, creating a false lumen. Most of these tears take place in the ascending aorta, usually in the right lateral wall where the greatest shear force on the aorta occurs. An AAD can propagate anterograde and/or retrograde and depending on the direction the dissection travels, cause branch obstruction that produces ischemia of affected territory (coronary, cerebral, spinal, or visceral), and for proximal type A AADs can instigate acute tamponade, aortic regurgitation or aortic rupture.[6]
In an AAD, the true lumen is lined by the intima whereas the false lumen is within the media. In most cases, the true lumen is smaller than the false lumen. Overtimes, the blood flowing through the false lumen leads to the development of an aneurysm with the potential for rupture. The three common sites for AAD are as follows:
- Nearly 2-2.5 cm above the aortic root (the most common site)
- Just distal to the origin of the left subclavian artery
- In the aortic arch
Histopathology
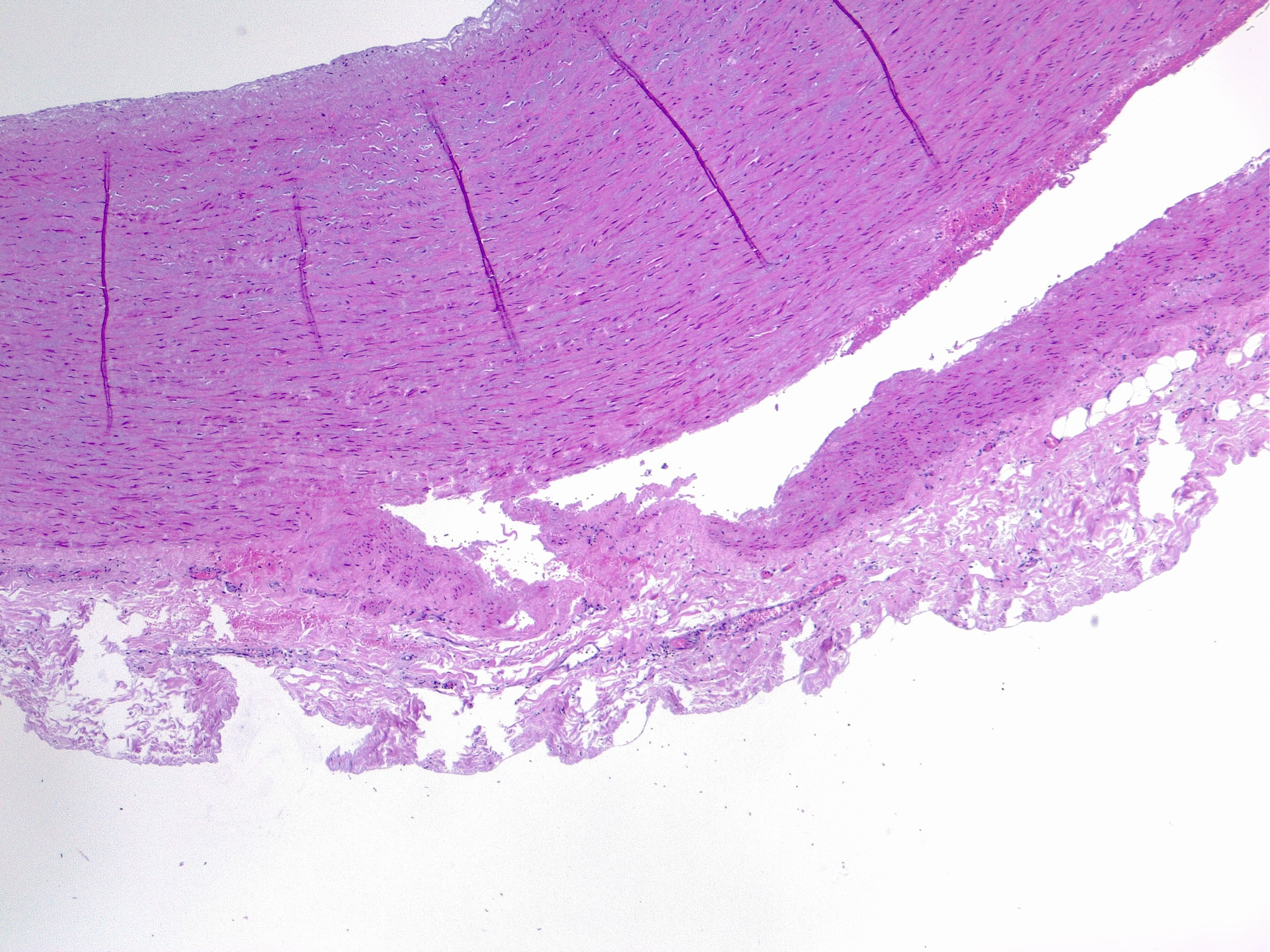
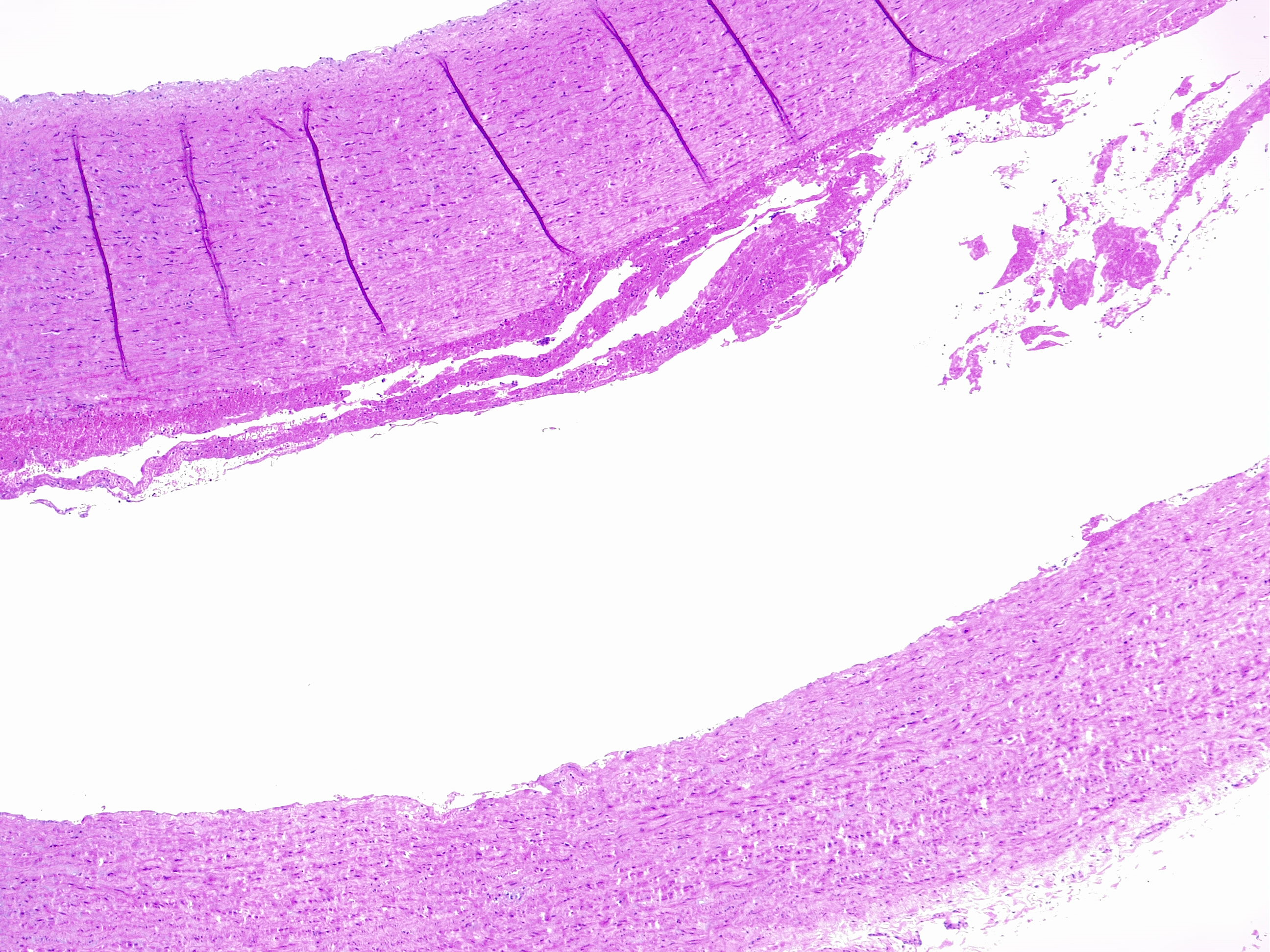
Aortic dissection is an acute process in which a tear in the internal face of the aorta leads to dissection through the laminas and formation of a new lumen (false lumen) and acute drop in systemic blood pressure, potentially leading to hemopericardium and cardiac tamponade [7] with sudden death. This is the endpoint of a long process of injury and repair within the aortic wall, usually due to hemodynamic forces such as chronic systemic hypertension. The tear usually dissects the media in two parts, forming a lumen between intima-media and media-adventitia. More common in this setting is the dissection in the outer one-third of the media [8]. Therefore fragility of this layer decreased aortic distensibility and has the potential for aortic dissection.
The media layer is formed by smooth muscle cells, elastic fibers, collagen fibers, and hyaluronic acid. Each of these components can be altered and weaken the structure leading to a less resistant vessel. Microscopic examination can evaluate each component with special stains: VVG, Alcian blue, Movat's pentachrome, Masson's trichrome, CME.
- Extracellular matrix: mucoid accumulation increase the intralamellar or translamellar space, creating the so-called "cystic degeneration" of the media that decreases the vessel resistance. The main component of mucin accumulation is chondroitin-6-sulfate [9].
- Alteration of elastic fibers: fragmentation, thinning, disorganization. A normal aorta has a dense meshwork of elastic fibers that contribute substantially to its resistance to blood pressure.Fragmentation of elastic fibers extends the translamellar spaces decreasing the elasticity of the aorta. Thinning widens the intralamellar spaces. Disorganization refers to the nonparallel arrangement of fibers that are haphazardly arranged in a non-functional manner.
- Altered smooth muscle cells: nuclear loss, disorganization.Nuclear cell loss, so-called “smooth muscle cell necrosis” can appear in bands or as wide areas where only the ghost cell contours are visible. Disarray of smooth muscle cells can be focal or nodular.
- Laminar medial collapse: collapsing of elastic fibers represent what is called “laminar medial necrosis” and is coupled with significant loss of interponed smooth muscle cells.
- Collagen fibers: fibrosis. This is the least common process in degenerative aortic disease.
Those findings are usually present combined with each other and must be recognized to accurately grade the medial degeneration (as mild/moderate/severe) from severity and distribution of the degenerative lesions [10][11].
Vasa Vasorum
The vasa vasorum is a vascular network distributed around the outer layers of the media and the adventitia of the aorta. Its role is crucial in maintaining the oxygen and nutrient supply to the aorta itself. Reduced vascular efficacy of the vasa vasorum leads to medial alteration and necrosis. As proven empirically, obstruction of the vasa vasorum induces degenerative changes in the media with subsequent aortic dissection[17]. This can be physiologically caused by vascular thickening, tortuosity, and obstruction.
Hypertension
It is the single most important risk factor for the development of medial degeneration[12][13]. Hypertension causes altered hemodynamic forces acting on the aortic wall and causes direct damage to the aortic media, intimal thickening, and consequent aortic stiffness.
Aging
With aging, there is a loss of elastin and an increase in collagen, fragmentation, and disarray of elastic fibers with different grades of the smooth muscle cell alterations[14]. This is the main physiological reason for the high epidemiological correlation between age and aortic diseases such as aneurysms and dissection [9]. Also, fibrosis is often accompanied by microcalcification in the inner acellular portion of the media in elderly people.
Genetic Predisposition
Marfan syndrome: in this genetic syndrome the fiber meshworks are altered, and the vessels are more prone to aneurysm formation.Other syndromes are associated with an altered aortic wall: Ehlers-Danlos, Loeys–Dietz syndrome (LDS), and Turner syndrome.
Congenital diseases
Tetralogy of Fallot, coarctation of the aorta, bicuspid aortic valve.
History and Physical
Clinically, the presentation of AAD is subject to the extent of the dissection, with complaints corresponding to those affected cardiovascular structures. Three fundamental questions that must be addressed when taking a history of a patient with suspected AAD are the quality, radiation, and intensity of pain at the onset. Various studies identified the intensity of onset of pain as the most reliable historical factor. While increasing the probability of AAD when present, classically cited physical findings, such as a discrepancy of blood pressures in the upper extremities, a pulse deficit, or presence of a diastolic murmur, are present in less than 50% of confirmed cases of AAD. Additionally, the presence of chest pain with any neurological finding, the combination of chest and abdominal pain, or chest pain accompanied by limb weakness or paresthesia should alert the clinician to the possibility of AAD.
The pain of AAD is often sudden in onset, reaches maximal severity quickly, and can be tearing in nature. In about 10% of patients, the AAD is painless, which is more common in Marfan syndrome. The pain can be located in the anterior chest in case of ascending aorta and in the back if the dissection is descending. It may have a migrating character as dissection propagates caudally.
At the time of presentation, neurological deficits are present in 1/5th of patients. Syncope is also common and may be due to hypovolemia, arrhythmias, MI, or an increased vagal tone. If the dissection is antegrade, it may involve the extremity vessels leading to loss of pulses, paresthesias, and pain.
Hoarseness and Horner syndrome have also been described in patients with AAD.
If the AAD is leaking or ruptured in the mediastinum, then the patient may have dyspnea and hemoptysis.
Physical findings
Hypertension is very common in AAD; if the patient presents with hypotension then this is a grave sign most likely indicating a rupture. A difference of more than 20mmHg in blood pressure between the arms should raise suspicion of AAD. Other features include:
- Wide pulse pressure
- Aortic insufficiency
- Diastolic murmur
- Muffled heart sounds (suggesting cardiac tamponade)
- Syncope
- Altered mental status
- Loss of peripheral pulses
- Horner syndrome
Evaluation
Routine studies like an ECG and chest x-ray can help differentiate other possible causes for chest pain but can be misleading. The presence of ECG findings consistent with an acute myocardial infarction occurs in eight percent of cases of AAD. Furthermore, while the widening of the aortic silhouette increases the likelihood of AAD, its absence does not reliably exclude the diagnosis. Confirmation of AAD requires cardiovascular imaging to identify the presence of an intimal tear, establish the Stanford classification, and detect valvular or branch involvement. Most society guidelines recommend either CT aortography or transesophageal echocardiogram (TEE) for the diagnosis of AAD. Deciding which modality to employ as a first-line screen should be based on institutional availability and expertise. For most emergency departments (EDs), CT angiography will likely be the first advanced imaging technique on account of its widespread availability.[15]
Blood work should include CBC, electrolytes, troponin, D-dimer, and renal function. Smooth muscle myosin heavy chain assay is elevated is specific for AAD.
The chest x-ray may show a widened mediastinum (>8cm) or fluid collection in the hemithorax due to rupture. X-ray features that suggest AAD include:
- Left apical cap
- Pleural effusion
- Deviation of the esophagus
- Deviation of the trachea to the right
- Depression of the left mainstem bronchus
- Loss of the paratracheal stripe
If the patient is stable, a CT scan with contrast is recommended. The spiral CT can quickly detect the location of the intimal tear and help the surgeon plan the procedure. Suggestive of AAD findings include:
- Intimal dissection flap
- Double lumen
- Aortic dilation and hematoma
- Regions of malperfusion
- Contrast leak indicates aortic rupture
Echocardiography is also a useful modality to detect AAD. TEE is the preferred technique that can be done at the bedside and used for intraoperative visualization. The key drawback is the operator experience. However, if the patient is unstable, TEE is the recommended procedure. Other indications for TEE include renal insufficiency and contrast allergy. Findings may include:
- Dissection flap with differential Doppler flow
- True and false lumen in the ascending aorta
- Thrombosis in the false lumen
- Central displacement of intimal calcification
- Pericardial effusion
Aortography is not much used today unless there are plans to repair the dissection using stents.
A 12 lead ECG is mandatory to rule out an MI.
Treatment / Management
Once the diagnosis of AAD is confirmed or highly suspected, urgent cardiothoracic or vascular surgery consultation should be obtained, regardless of the dissection location. Acute dissections involving the ascending aorta are considered surgical emergencies. The patient should have an arterial line and central venous catheter for monitoring. A foley should be inserted to assess urine output.
Concurrently commence medical therapy including providing adequate analgesia (morphine is the preferred analgesic, as it decreases sympathetic output as well) and administering a short-acting IV beta-blockers aiming for a heart rate of ~60 bpm (reductions in heart rate and blood pressure reduces aortic wall tension and limit the extent of dissection). In patients with contraindications to b-blockade non-dihydropyridine calcium channel blockers can be used for rate control. If the systolic blood pressure remains elevated, nitroprusside can be added to achieve a systolic blood pressure goal of 100 to 120 mmHg (maintain blood pressure in this range as long as there is no compromise of mentation or urine output). Other agents that can be used to lower blood pressure include esmolol, labetalol, or diltiazem. B-blockers should be used with caution in the settings of acute aortic regurgitation because they block compensatory tachycardia.
In hypotensive patients, intravenous fluids administration is a reasonable first approach. Vasopressors can be added, if needed, to maintain adequate perfusion but may cause further false lumen propagation. Inotropic agents should be avoided as they are likely to increase the force and rate of ventricular contraction and therefore worsen aortic wall stress.
Surgical therapy for type A AAD involves excision of the intimal tear, obliteration of entry into the false lumen proximally, and reconstitution of the aorta with the interposition of a synthetic vascular graft. Surgical intervention for type B AAD tends to be reserved for patients who have a complicated course. Endovascular stent-grafting (TEVAR) has been employed as a less invasive alternative to surgery, primarily for patients with complicated type B dissections.[2][16][17]
For AAD involving the ascending aorta, the aorta has to be replaced and the valve has to be assessed. In most cases, the aortic valve may also have to be excised and replaced with a prosthetic valve. Dissections involving the descending aorta are complex and there is a risk of paraplegia. The most difficult dissections are those that involve the aortic arch. Surgical mortality varies from 5-20%. Even those who survive have a long recovery.
Because of the high surgical mortality, today endovascular procedures are being performed for AAD. Endovascular stent placement is associated with significantly lower morbidity compared to surgery but the key is patient selection.
Differential Diagnosis
Alternative diagnoses on the differential of aortic dissection include the following life-threatening conditions: myocardial infarction, aortic aneurysm, cardiac tamponade (from another cause), esophageal rupture (Boerhaave syndrome), spontaneous pneumothorax, pulmonary embolism, and stroke / transient ischemic attack. If a patient presents with abdominal pain, renal or biliary colic, bowel obstruction/perforation, non-dissection-related mesenteric ischemia should be considered. Pulse deficit can be a sign of non-dissection-related embolic phenomena or arterial occlusion. [18]
Prognosis
Aortic dissection still carries a very high mortality. At least 30% of patients die after reaching the emergency room and even after surgery, the mortality rates vary from 20-30%. For those who survive the surgery, the comorbidity also takes a toll and the quality of life is poor. the highest mortality of an acute aortic dissection is within the first 10 days. Patients with a chronic dissection tend to have a better prognosis, but even their life expectancy is shortened compared to the general population.
Complications
- Multiorgan failure
- Stroke
- MI
- Paraplegia
- Renal failure
- Amputation of extremities
- Bowel ischemia
- Tamponade
- Acute aortic regurgitation
- Compression of superior vena cava
- Death
Postoperative and Rehabilitation Care
- Once the patient is treated surgically or medically, the blood pressure must be controlled
- The patient must be closely monitored for the progression of the aortic dissection
- A regular CT scan of the chest or MRI is recommended at 3-6 month intervals to check for the progression of the disease
Deterrence and Patient Education
- Controlling blood pressure
- Avoid the use of illicit drugs
- Maintain a healthy weight
- Discontinue smoking
Pearls and Other Issues
Despite the best of circumstances, it is next to impossible to diagnose every case of AAD presenting to the ED. Factors contributing to a high miss rate include perceived mildness of symptoms in some patients with AAD. A second factor leading to misdiagnosis is clinical symptoms and laboratory findings suggesting an alternative diagnosis such as acute coronary syndrome (ACS). A third factor was the lack of expected results such as a pulse deficit or absence of a widened mediastinum on chest x-ray. Approach every chest pain patient as if they could harbor an ADD, establish a risk factor profile, and remain cognizant of more subtle presentations.
Enhancing Healthcare Team Outcomes
Aortic dissection is a medical emergency with very high mortality if it is undiagnosed. The majority of patients present to the emergency department and hence an interprofessional team is the key.
Once the patent with an aortic dissection presents to the emergency room, a standardized system must be in operation to ensure that the diagnosis and management are done without any delay. The triage nurse should be fully aware of the importance of immediate admission of the patient and consult with the emergency physician and cardiac surgeon. The decision on how to make the diagnosis depends on patient stability and the availability of imaging tests. The two options include an ECHO or a CT scan. An unstable patient should never be sent to the radiology suite. Instead, a cardiologist should be consulted for a bedside echo. The intensivist should be notified as there is an urgent need to lower blood pressure. The operating room should be notified that a patient with a dissection has been admitted. Nurses should be fully aware of the potential complications of aortic dissection and know what medications to use to lower the blood pressure. In addition, nurses should monitor fluid input and output and keep the patient NPO. After surgery, the nurses need to closely monitor the patient for complications. Open communication between the team members is vital to improving outcomes. [17][19][20](Level V)
Outcomes
Several studies show that patient outcomes are improved when managed by an interprofessional team of healthcare professionals that include a cardiologist, intensivist, pulmonologist, nephrologist, cardiac surgeon, interventional radiologist, and anesthesiologist. In addition, the pharmacist must educate the patient about the importance of blood pressure control and compliance with medications. The outcomes of aortic dissection tend to be better in high volume centers compared to small centers which do less than 5 cases a year. [21][22][23](Level V)