Continuing Education Activity
Pityriasis rosea, also known as pityriasis circinata, roseola annulata, and herpes tonsurans maculosus, is an acute self-limiting papulosquamous disorder. The hallmark features of this condition include the development of a slightly raised, oval-shaped scaly patch called a "herald patch," followed by the emergence of multiple clusters of similar scaly oval patches within 2 weeks. These clusters typically distribute in a "Christmas tree" pattern on the trunk and proximal extremities. Pityriasis rosea (PR) can sometimes be difficult to manage because of factors including variations in the clinical manifestation of the condition and acknowledging instances where the typical herald patch may be absent. The exact cause of PR is unknown; however, features like seasonal variation and community clustering suggest that it is an infectious disease. Infections like viruses, bacteria, and spirochetes, as well as noninfective causes like atopy and autoimmunity, are known causes of PR. Upper respiratory tract infections that precede PR suggest that streptococcus plays a role in developing this condition. Recently, reactivation of latent human herpesvirus (HHV)-6 and HHV-7 infections have been identified as possible etiologic agents. This activity aims to empower healthcare professionals with the necessary tools to recognize, evaluate, and manage pityriasis rosea, highlighting the significance of an interprofessional approach in optimizing patient outcomes.
Objectives:
Identify the characteristic skin presentations of pityriasis rosea (PR).
Compare all the investigation options available for PR.
Apply current evidence-based treatment options for PR.
Implement interprofessional team strategies for improving care, coordination, and communication for patients with PR.
Introduction
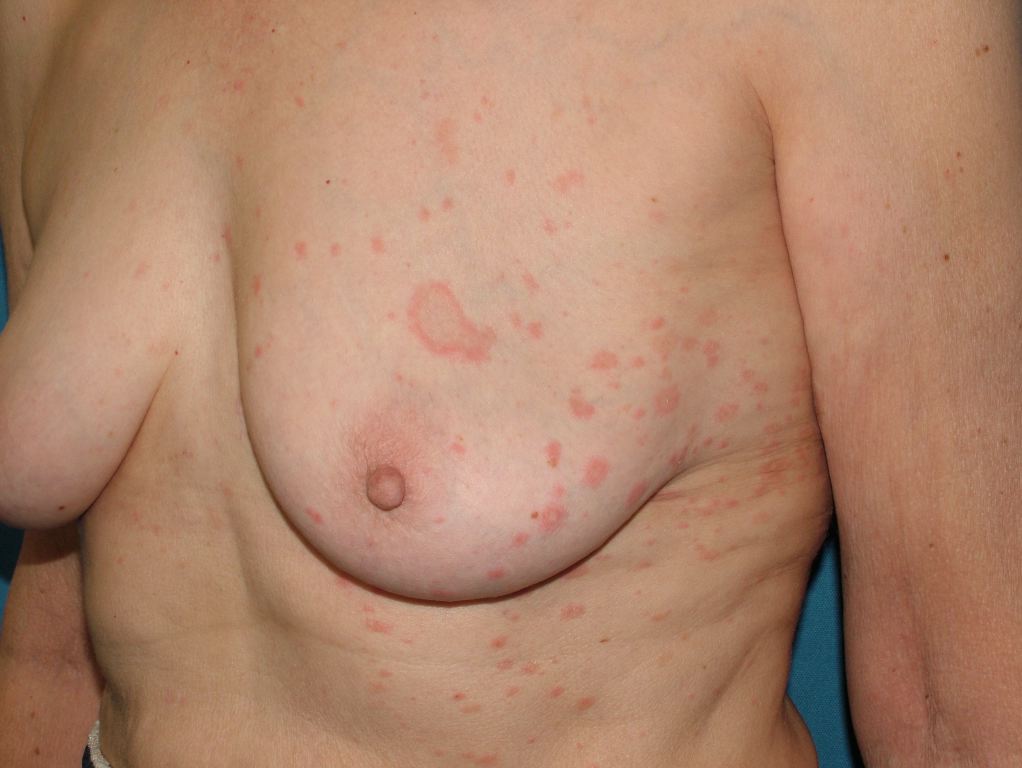
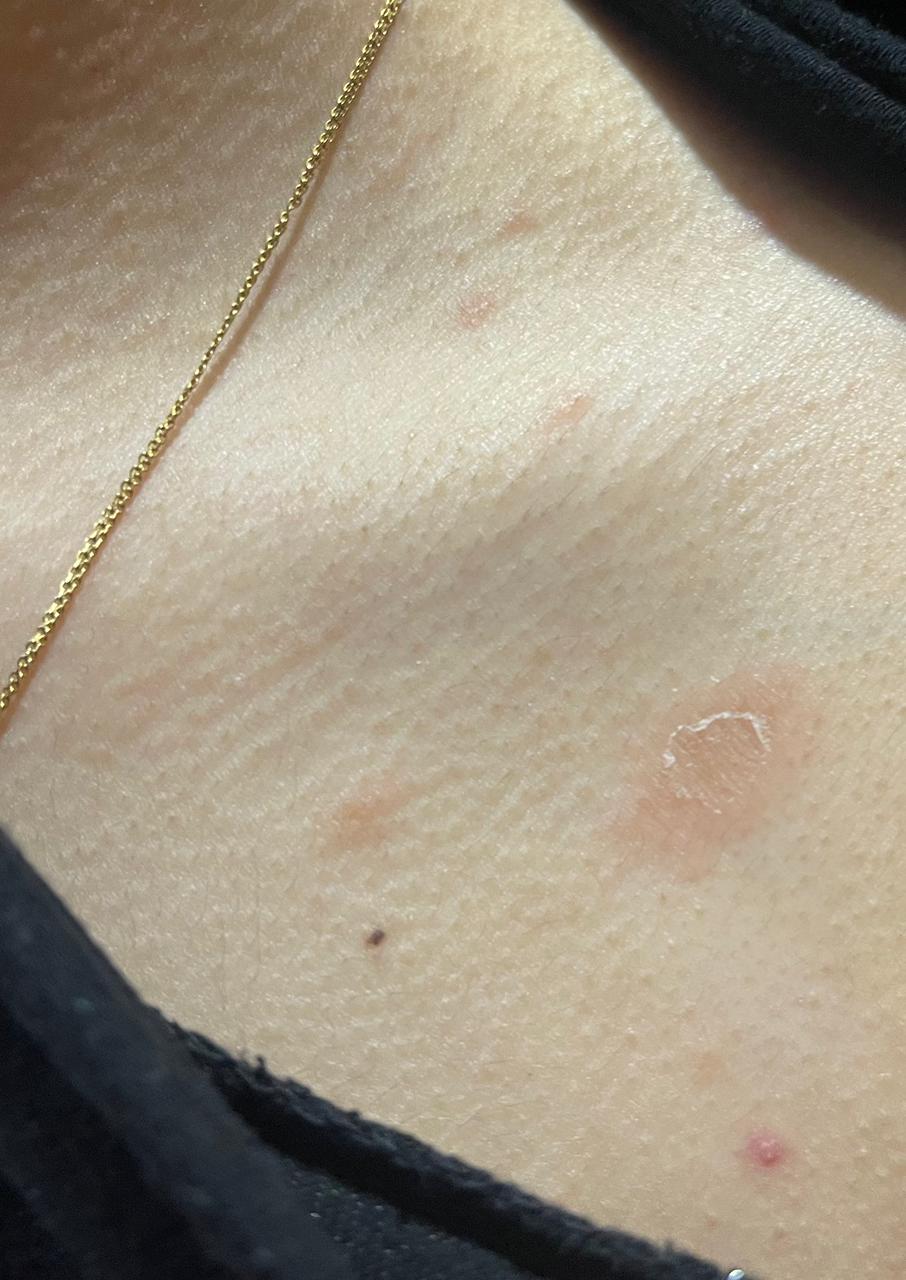
Pityriasis rosea (PR), meaning rose-colored scale, is an acute self-limiting papulosquamous disorder. [1] Also known as pityriasis circinata, roseola annulata, and herpes tonsurans maculosus, PR is often distinguished by the development of a slightly raised, oval, scaly patch called the "herald patch." It is later followed by multiple clusters of scaly oval patches on the trunk and proximal extremities in a "Christmas tree" appearance. The skin eruption usually lasts 6 to 8 weeks and although it usually begins with a pathognomic herald patch, some cases are reported that may not exhibit this characteristic finding. [2][3][4]
Etiology
The exact cause of PR is unknown; however, features like seasonal variation and community clustering suggest that it has an infection-based origin. Infections from viruses, bacteria, and spirochetes, as well as noninfective causes like atopy and autoimmunity, are known causes of PR. [5][6] Upper respiratory tract infections that precede PR suggest that streptococcus plays a role in developing this condition. Recently, reactivation of latent human herpesvirus (HHV)-6 and HHV-7 infections have been identified as possible etiologic agents. [7]
PR-like eruptions have been reported after vaccinations, such as bacillus Calmette-Guérin (BCG), influenza, influenza A (H1N1), diphtheria, smallpox, hepatitis B, pneumococcus, and COVID-19. [8] Eruptions have also been seen after administration of drugs like gold compounds, captopril, barbiturates, D-penicillamine, clonidine, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs, hydrochlorothiazide, atypical antipsychotics, imatinib, metronidazole, isotretinoin, and clozapine. [9] In temperate regions, PR is more common in the winter, whereas in tropical areas, some seasonal variation is noted. [10][11][12]
Epidemiology
The approximate incidence of PR is 0.5% to 2%. The condition affects men and women, but studies reveal it is more commonly seen in women. [13] Adolescents and adults between 15 and 30 are most frequently affected, but PR also affects older adults and children. [14][15] More cases of PR are seen in the monsoon and fall seasons. [16]
Pathophysiology
The pathophysiology of PR is not entirely understood. However, a lack of natural killer (NK) cell and B-cell activity in PR lesions has been noted, suggesting a predominantly T-cell–mediated immunity. Increased CD4 T-cells and Langerhans cells are present in the dermis, possibly reflecting viral antigen processing and presentation. [17] Anti-immunoglobulin M (IgM) keratinocytes have been found in patients with PR. This may be associated with the exanthem phase of viral infection. [18]
Histopathology
Although skin biopsy is not necessary to diagnose pityriasis, when performed, it usually reveals many nonspecific features similar to those seen in chronic dermatitis. Characteristic features include the absence or regression of the granular cell layer and extravasation of red blood cells in the papillary dermis and partly into the epidermis. Dyskeratosis, liquefaction of basal cells, papillary collagen homogenization, and intraepidermal vesicles are also evident. Notably, biopsies from herald patches show minor differences from the secondary lesions. [19]
History and Physical
PR is characterized clinically by a herald patch, or mother patch, followed by scaly oval plaques on the trunk and proximal extremities along the Langer lines of cleavage, giving a characteristic "Christmas tree" appearance. Collarette scaling is common. Pruritus is severe in 25% of cases. The herald patch is seen in 50% to 90% of patients and is located on the trunk, followed by the neck or proximal extremity. Over 1 to 2 weeks after the onset of the herald patch, a generalized skin eruption occurs, in which numerous lesions develop in crops. The eruption is usually preceded by a prodrome of a sore throat, gastrointestinal disturbance, fever, and arthralgia. [20] The resulting lesions are symmetric and most commonly involve the thorax, back, abdomen, and adjoining areas of the neck and extremities.
These secondary lesions occur as macules and papules that are elliptical or ovular in shape. Fine scaling and central wrinkling with a cigarette-paper aspect are seen. A characteristic feature of PR is the collarette appearance of the scale, with edges peripherally attached and lifted near the center of the lesion. The distribution of the lesions is usually bilateral and diffuse, with the long axis running parallel to skin tension lines.
The incidence of atypical PR is 20%, which may be due to the morphology, size, and distribution of lesions, as well as the symptoms or course of the condition. The various atypical morphologies include: [21]
- Vesicular PR: This condition presents as a generalized eruption of 2- to 6-mm vesicles or as a rosette of vesicles, mainly over the head, palms, and soles; it is commonly seen in children and needs to be differentiated from varicella and dyshidrosis.
- Purpuric (hemorrhagic) PR: This condition presents as macular purpura on the skin or oral mucosa.
- Generalized papular PR: This condition presents as multiple papules that occur along with classic patches and plaques; it is seen in young children, pregnant women, and African Caribbeans.
- Lichenoid PR: This condition is observed in the course of atypical PR, but it is more commonly caused by drugs such as gold compounds, captopril, barbiturates, D-penicillamine, and clonidine.
- Erythema multiforme–like PR: This condition presents with targetoid lesions along with the classical lesions of PR. Histopathologically, erythema multiform and PR may show similar features except for satellite cell necrosis, a distinguishing feature seen only in erythema multiform where lymphocytes are attached to scattered necrotic keratinocytes.
- Follicular PR: The secondary lesions of this condition are typically follicular and present discretely or in groups associated with classical lesions. Differential diagnoses include follicular lichen planus, keratosis pilaris, and atopic dermatitis with a follicular element.
- Giant PR: This condition consists of plaques and circles of very large sizes ranging from 5 to 7 cm, with individual lesions reaching the size of the patient's palm; it was named after Darierand and is rarely reported.
- PR presenting as exfoliative dermatitis
- PR with atypical herald patch: The herald patch may be absent in 20% of patients; alternately, this condition may present with secondary eruptions or occur at unusual sites such as the face, scalp, genitalia, or other sites.
- Inverse PR: In this condition, lesions are predominantly present in acral and flexural areas involving the axilla, groin, and face. [22]
- Acral PR: In this condition, lesions are more concentrated over acral parts of the body (ie, palms and soles), where erythema multiforme, syphilis, necrolytic acral erythema, and drug eruptions should be kept as differentials.
- Unilateral PR: This rare variant of PR can be seen in both children and adults; lesions are located on one side of the body, and patients exhibit a herald patch with classical secondary lesions. [23]
- Blaschkoid PR: In this condition, the PR lesions follow the lines of Blaschko. [24]
- Limb-girdle PR: Also known as PR of Vidal, eruptions in this condition are limited to the shoulders or pelvic girdle, thus involving the axilla and groin; lesions are usually larger and more annular.
- Mucosal involvement in PR: This variant is seen in 16% of patients with PR; it affects the oral mucosa, with punctuate, erosive, bullous, hemorrhages, ulcers (with or without raised borders), petechiae, papulovesicular, bullae, and erythematous plaques.
- Localized PR: In this condition, eruptions are localized to one part of the body.
The rashes of PR usually last for 5 weeks and resolve by 8 weeks in more than 80% of patients. PR needs to be differentiated from secondary syphilis, dermatophytosis, guttate psoriasis, nummular eczema, pityriasis lichenoid chronic, cutaneous T-cell lymphoma, and erythema annular centrifugal and erythema chronic migrans.
Evaluation
Dermatoscopy helps differentiate PR from other conditions. This technique shows a yellowish background color, a peripheral arrangement of the scales, and a patchy distribution of loosely arranged dotted vessels. [25]
Histopathology shows superficial perivascular dermatitis. Focal parakeratosis in mounds, hyperplasia, and focal spongiosis are observed in the epidermis. The epidermis may also show exocytosis of lymphocytes, variable spongiosis, mild acanthosis, and a thinned granular layer. Extravasated red blood cells and a perivascular infiltrate of lymphocytes, histiocytes, and eosinophils are seen in the dermis. [3][26][27]
Treatment / Management
PR is a self-limiting, exanthematous disease. Other than general measures like using moisturizers, bathing with soap alternatives, and cautiously exposing skin to sunlight without burning, some specific treatments may also be used. Most patients respond to emollients, antihistamines, and topical steroids. Macrolides and acyclovir lead to faster resolution of lesions and help to relieve pruritus. [4] Narrowband ultraviolet B therapy is also used; it alters the immune response in the skin and has shown favorable results in patients. [28]
Differential Diagnosis
Lesions of PR resemble and must be differentiated from:
- Erythema multiforme
- Guttate psoriasis
- Kaposi sarcoma
- Lichen planus
- Parapsoriasis
- Pediatric syphilis
- Pityriasis alba
- Seborrheic dermatitis
- Tinea corporis
- Tinea versicolor
Prognosis
Pityriasis rosea typically follows a self-limiting and benign clinical course. The condition commonly resolves spontaneously within weeks to a few months. Symptomatically, mild-to-moderate itching might accompany the rash, but the overall impact on health is minimal for most individuals. Recurrence is rare (< 5%), with most cases presenting as a singular occurrence in a person's lifetime. Complications such as secondary bacterial infections due to scratching are infrequent. While certain systemic associations have been proposed, they remain less common. Timely diagnosis by a healthcare professional ensures appropriate guidance and reassurance, considering the generally favorable prognosis of pityriasis rosea. [29]
Complications
PR usually resolves on its own within a few weeks or months without serious complications; however, in rare cases, some people may experience complications, including:
- Superimposed bacterial infection: Scratching the involved skin area can lead to a secondary bacterial infection, which can cause additional symptoms such as pain, redness, and pus.
- Itching, discomfort, and impaired quality of life: PR can cause intense pruritus, especially during the early stages of the condition, which can cause sleep disturbances and affect quality of life.
- Hyperpigmentation: In some cases, the rash may leave behind darkened patches of skin that can take several months to fade.
- Recurrence: Although uncommon, some people may experience a recurrence of the rash, usually within the first few months after the initial episode.
Other rare complications seen in a few reported cases include premature delivery and fetal demise during pregnancy (within the first trimester up to 15 weeks of gestation) and drug hypersensitivity reaction due to reactivation of HHV-6 and HHV-7.
Deterrence and Patient Education
Deterrence measures for PR include avoiding close contact with infected individuals and maintaining good hygiene practices, which can help reduce the spread of the condition and prevent new cases.
Patient education is also an essential aspect of managing PR. Patients should be educated about the nature of the condition, its expected course, and the need for symptomatic relief. Patients should be informed that the condition is usually self-limiting and that the rash will resolve without specific treatment. However, symptomatic relief can be achieved through topical corticosteroids, antihistamines, and moisturizers.
Patients should be advised to avoid harsh soaps, hot water, and excessive sun exposure, as these can exacerbate the rash and delay healing. It is also advisable that patients wear loose-fitting clothing and avoid activities that may cause excessive sweating, as this can also worsen the rash.
Lastly, patients should be urged to seek medical attention if they experience severe symptoms, such as severe itching, pain, or fever. In such cases, further investigation may be necessary to rule out other underlying conditions that may mimic the physical presentation of PR.
Enhancing Healthcare Team Outcomes
In the majority of patients, PR is a self-limited condition with an excellent prognosis; however, about 2%-3% of patients will experience a recurrence. The skin disorder is benign, noncontagious, and does not require any special precautions. Since most patients first present to their primary care provider when the skin lesions appear, nurses, pharmacists, and primary care providers should inform patients that the condition is benign and does not last for more than 2 months.
For severe cases, a dermatology referral should be made. The main morbidity is due to pigmentation changes, especially in dark-skinned individuals; however, scarring does not occur. There are reports that PR during pregnancy may be associated with premature birth, but it is not known if this is just a coincidental observation. Patients should be advised to avoid applying irritants to the skin and avoid tanning. The itching that accompanies PR is mild and usually resolves with a moisturizer. Exposure to the sun may induce pigmentary changes and should be avoided. [30][31]