Continuing Education Activity
Spinal cord injuries (SCIs) are complex medical conditions resulting from spinal cord damage, often caused by trauma, as in motor vehicular crashes and falls, and nontraumatic etiologies like malignancy and degeneration. Spinal cord lesions may result in severe morbidity and permanent disability. High spinal lesions are often a cause for emergency due to cardiorespiratory compromise.
The pathologic mechanisms causing SCIs are classified as either primary or secondary. Primary injury, often irreversible, arises from direct spinal cord damage. Secondary injury occurs as a consequence of the changes induced by a primary injury, such as inflammation. Nerve axon disruption produces motor and sensory function loss below the level of injury. The diagnosis depends on a combination of clinical examination and diagnostic testing, particularly imaging studies. Treatment options include conservative, operative, rehabilitative, or a combination of these modalities. Novel interventions like stem cell therapy are still under investigation.
This activity for healthcare workers is designed to enhance learners' proficiency in evaluating and managing SCIs. After participation, learners strengthen their skills in utilizing best practice guidelines, implementing emergency measures, and seeking consults within the appropriate timeframes. Participants heighten their diagnostic and management acumen, enabling them to collaborate effectively within an interprofessional team seeking to improve outcomes for individuals with SCIs.
Objectives:
Compare the spinal cord syndromes based on pathophysiology and clinical features.
Create a clinically guided diagnostic strategy for an individual with suspected spinal cord injury.
Apply evidence-based practices when determining a personalized management plan for a patient diagnosed with a spinal cord injury.
Implement interprofessional team strategies for improving care coordination to advance the management of patients with spinal cord injuries.
Introduction
Spinal cord injury (SCI) is a multidimensional disorder arising from direct or indirect spinal cord damage. The most common SCI cause is acute trauma from motor vehicular crashes (MVCs), although the condition may also arise from insidious etiologies such as malignancies and chronic tuberculous infection. Spinal cord lesions may lead to permanent disability, significant morbidity, and even mortality. High spinal injuries often impair cardiorespiratory function, requiring emergent interventions.
Nerve axon disruption results in motor and sensory function loss below the SCI level.[1] SCIs disproportionately affect people younger than 30, leading to significant lifelong functional impairment and possibly causing numerous health, financial, and psychosocial complications.[2] SCIs are estimated to have a lifetime economic impact of $2 to 4 billion.[3][4] The interventions range from initial emergency stabilization to stem cell therapies requiring long-term investment. The therapeutic approach is, therefore, important for clinicians along a vast medical care spectrum.
Spinal Cord Anatomy
The spinal cord is a cylindrical nerve fiber bundle extending from the brain's base down through the vertebral column. The cord transmits sensory and motor signals between the brain and the rest of the body. The spinal cord is divided into segments corresponding to the vertebral levels. Each segment gives rise to spinal nerves supplying specific body regions. The key structures within the spinal cord include the corticospinal (CST) and spinothalamic (STT) tracts and the dorsal columns (DC)—nerve pathways through which information exchange between the brain and body occurs.
The CST is a major motor pathway responsible for voluntary movement. This nerve tract originates from the cerebral cortex's motor areas and descends through the brainstem and spinal cord. About 90% of the CST fibers travel on the spinal cord's lateral side, forming the lateral CST (LCST). The LCST nerves travel throughout the cord. The rest of the fibers transit ventrally or anteriorly, forming the ventral CST (VCST). However, VCST fibers do not reach levels below the superior thoracic spinal segments.
The STT is a sensory pathway that relays pain and temperature information from the body to the brain. This nerve tract ascends through the anterolateral spinal cord and synapses in the thalamus before projecting to the somatosensory cortex.
The DC, also known as the posterior columns or dorsal funiculi, carry proprioceptive and tactile sensations (ie, touch, pressure, vibration) from the body to the brain. DC fibers ascend in the spinal cord's posterior area and consist of the fasciculus gracilis (medial) and the fasciculus cuneatus (lateral).
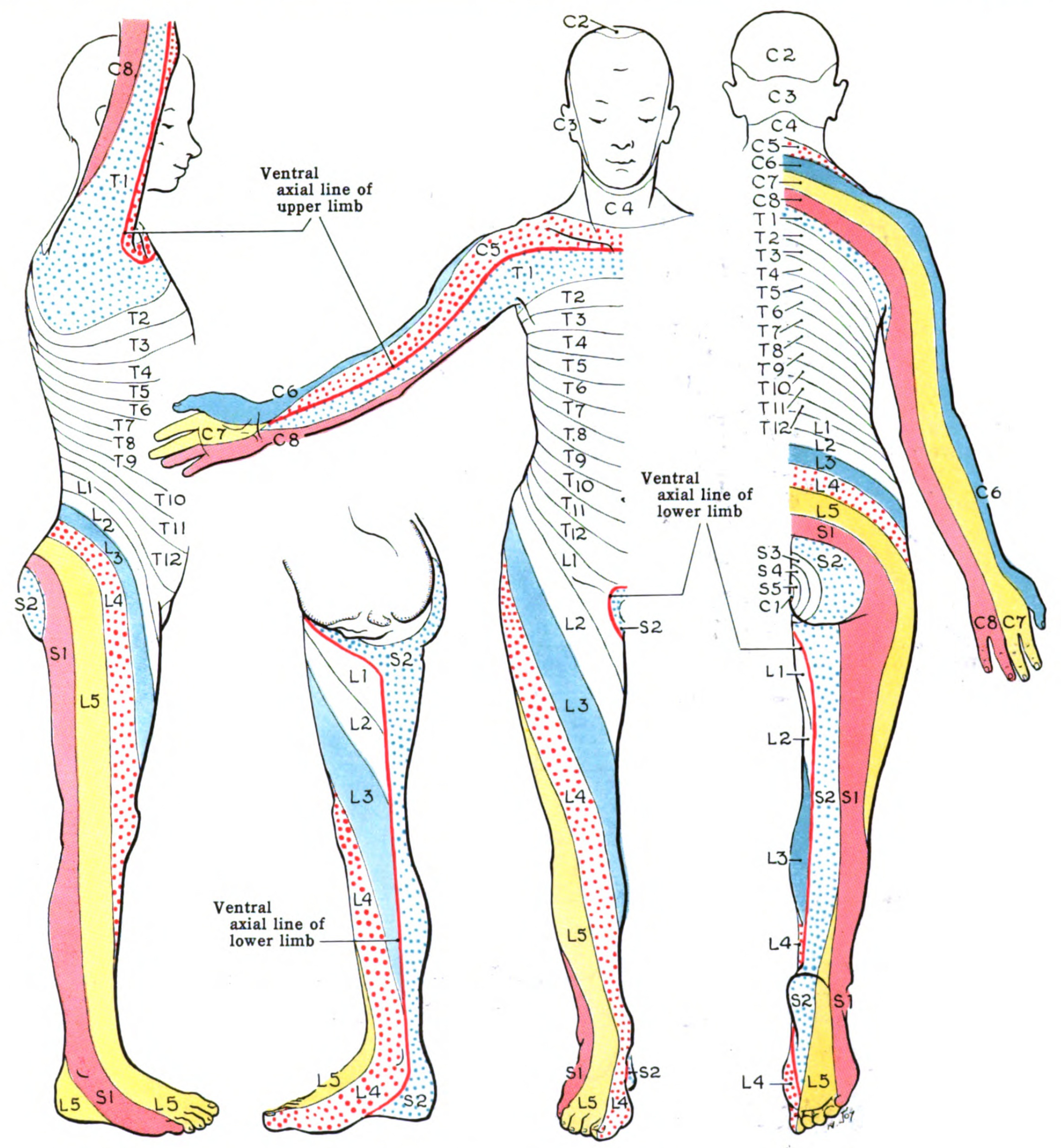
The spinal cord contains several distinct regions based on the vertebral segment levels. Cervical nerves C1 to C8 supply the neck, shoulders, arms, and hands. Thoracic nerves T1 to T12 innervate the upper limb's ulnar side and the trunk and abdominal muscles. Lumbar nerves L1 to L5 supply the lower back, buttocks, and lower limbs. Sacral nerves S1 to S5 innervate the pelvic organs, buttocks, genitals, and lower limbs. The coccygeal nerve C0 provides sensory innervation to the skin overlying the coccyx and surrounding areas and contributes to the pelvic floor muscles' motor function. Spinal nerve distributions are best represented by dermatomal maps (see Image. Dermatome Map).
The cauda equina consists of spinal nerve roots L2 to S5, extending from the spinal cord's lower end. The conus medullaris represents the spinal cord's terminal portion, typically located at the L1 to L2 levels. The filum terminale anchors the spinal cord and dural sac to the coccyx and extends from the conus medullaris, providing structural support to the cord.
SCIs give rise to a myriad of clinical manifestations, requiring different management strategies. Understanding the anatomy and organization of the spinal cord, including the locations of important nerve pathways, is essential for diagnosing and managing neurological conditions affecting motor and sensory function.
Etiology
The leading SCI cause in the United States is MVCs, constituting 38% of new injuries each year. Around 30% are due to falls, 13% are due to violence, 9% are from sports injuries, and 5% are from medical and surgical etiologies.
Epidemiology
Between 250,000 and 500,000 patients each year suffer from SCIs globally. Most SCI cases arise from preventable causes, such as violence and MVCs. Approximately 17,000 new SCI cases in the United States occur each year, and 282,000 persons are estimated to be living with SCIs. Most sports-related SCIs occur in male patients. The age group with the highest SCI risk is 16 to 30.
Pathophysiology
SCI Mechanisms
SCIs arise from complex mechanisms, producing varying degrees of neurologic deficits depending on the injury's location and extent. The processes driving SCIs are classified as either primary or secondary. The spinal cord lesions may give rise to unique, clinically identifiable syndromes. These concepts are further explained below.
Primary injury
A primary SCI develops from mechanical forces directly damaging the cord. The most common primary SCI mechanism is direct cord trauma, followed by persistent compression from space-occupying pathologies like vertebral fractures, malignancies, hematomas, and abscesses. Hyperextension injuries typically result from transient compression rather than impact alone, unlike fracture-dislocations.
Other common primary SCI mechanisms include distraction injuries and lacerations. A distraction injury arises from a spinal cord stretch and tear in its axial plane, typically when 2 adjacent vertebrae are pulled apart. A laceration or transection injury may come from sources such as sharp bone fragments, severe dislocations, and missile penetration.
Secondary injury
A secondary SCI emerges from a series of biological phenomena that begin within minutes of a primary injury and continue for weeks or months. The acute secondary injury phase encompasses vascular damage, ionic imbalances, free-radical formation, the initial inflammatory response, and neurotransmitter accumulation (excitotoxicity).[5]
SCI Phases
The phases of an SCI are summarized in the table below.
| Phase |
Timing and Duration After the Injury |
Characteristic features |
| Early acute phase |
First 2 to 48 hours |
- Increasing inflammation
- Edema
- Hemorrhage
|
| Acute phase |
First 2 hours to 2 weeks |
- Free radical generation
- Ionic dysregulation
- Excitotoxicity (due to glutamate-mediated pathways)
- Immune-related neurotoxicity
- Vascular disruption
- Progression of axonal injury and cellular necrosis
|
| Subacute phase |
Day 2 to end of week 2 |
- Phagocytic response to clear cellular debris
- Initiation of early axonal growth
- Damaged astrocytes undergo cellular edema and necrosis
- Peripherally located astrocytes proliferate and help to reestablish ionic hemostasis
- Blood-brain barrier restricts immune cell inflow
- Scar formation prevents axonal regeneration
|
| Intermediate phase |
Week 2 to month 6 |
- Continual maturation of the astrocytic scar
- Beginning of axonal sprouting
|
| Chronic phase |
Month 6 onward |
- Further scar maturation and the formation of syrinxes
- Wallerian degeneration persists
- Myelomalacia and cystic cavitations are formed [6]
|
Post-SCI Immune Response
Post-SCI neuroinflammation exhibits a dual nature, potentially causing both beneficial and deleterious outcomes, depending on the timing and immune cells present at the injury site. In the initial 3 days post-injury, blood-born neutrophils, resident microglia, and astrocytes are recruited to the injury site, initiating the inflammatory response. A second phase ensues around 3 days postinjury, attracting macrophages, and B and T lymphocytes. Antigen-presenting cells activate CD4+ helper T cells to release cytokines, stimulating B cells to produce antibodies and intensifying neuroinflammation and tissue destruction. Notably, neuroinflammation is most pronounced during the acute SCI phase.
Inflammation is protracted in the subacute and chronic phases. The inflammatory cell composition and phenotype vary with the inflammation stage and signal molecules within the injury microenvironment. T and B cells and microglia or macrophages can gain a pro-inflammatory or anti-inflammatory pro-regenerative phenotype. Spinal cord disruption leads to motor and sensory function deficits below the injury level. Disability patterns depend on the injury level and extent of spinal tract involvement.[7][8]
STT damage results in contralateral pain and temperature sensation loss. CST disruption leads to ipsilateral weakness or paralysis. In the cervical spine, CST fibers supplying the upper extremities are proximal to the center of the spinal cord. In contrast, CST nerves to the lower extremities are located distally. DC injury leads to contralateral loss of tactile, proprioceptive, and vibratory sensations.
Spinal Cord Syndromes
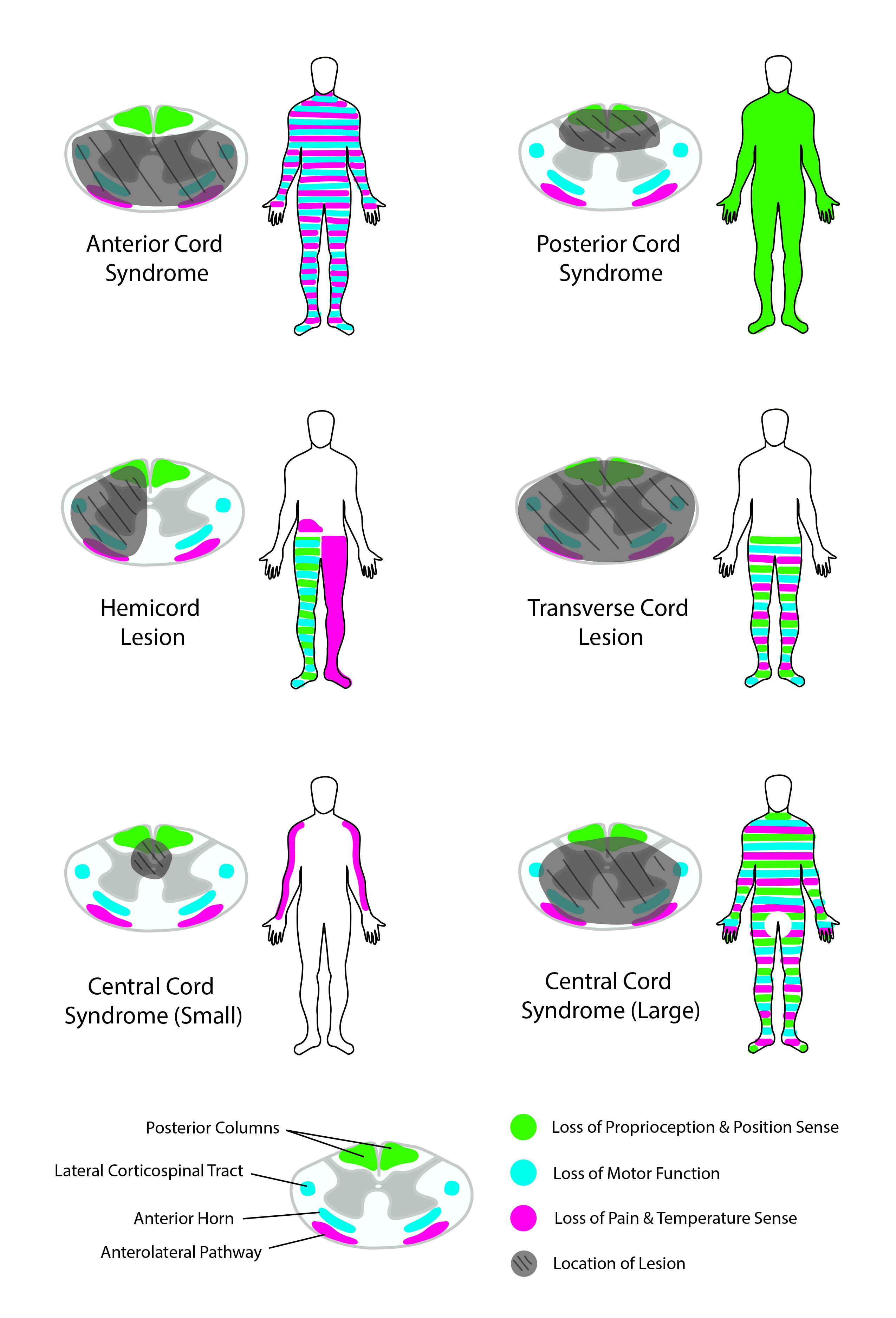
Several SCI patterns are well described (see Image. Spinal Cord Syndromes). The origins and manifestations of these different spinal cord syndromes are explained below.[7][8][9]
Complete spinal cord transection
Complete spinal cord transections typically demonstrate total bilateral loss of motor function and pain, temperature, proprioceptive, vibratory, and tactile perception below the injury level. Lumbosacral injuries present with lower extremity sensorimotor deficits and bowel, bladder, and sexual dysfunction. Thoracic injuries lead to the same deficits as lumbosacral injuries and, in addition, may result in torso motor weakness, producing postural difficulties. Cervical injuries lead to the same deficits as thoracic injuries but with added upper limb function loss, leading to tetraplegia. High-spinal injuries above C5 may also cause respiratory compromise due to loss of diaphragm innervation.[10]
Central cord syndrome
Central cord syndrome is the most frequently occurring incomplete SCI. This type of injury develops from neck hyperextension, thus compressing the cervical spinal cord and damaging the cord's center. This injury more often produces upper extremity than lower extremity weakness. This pattern emerges due to the CST's arrangement, where axons supplying the upper extremities are positioned closer to the spinal cord's center, while those serving the lower extremities are nearer the periphery. Pain and temperature sensation loss may be noted below the level of injury.
Anterior cord syndrome
This condition is classically due to compromised blood flow in the anterior spinal artery. Bilateral STT injuries lead to bilateral pain and temperature perception loss below the level of injury. Bilateral CST injuries paralyze the muscles below the injury level. Tactile and vibration sensations and proprioception remain intact, as dorsal columns are unaffected.
Posterior cord syndrome
This injury pattern occurs more frequently from infectious, toxic, or metabolic than traumatic causes. DC damage weakens tactile, vibratory, and proprioceptive perception. Pain and temperature sensory and motor function are preserved owing to the lack of STT and CST involvement.
Brown-Séquard syndrome
This condition results from right- or left-sided spinal cord hemisection. CST and DC transection lead to ipsilateral loss of motor function, proprioception, and tactile and vibratory sensations below the injury level. STT disruption produces contralateral pain and temperature perception deficits below the level of injury.[11]
Conus medullaris syndrome
This injury pattern develops from terminal spinal cord damage at an area proximal to the cauda equina. Conus medullary syndrome characteristically presents with sacral nerve dysfunction, manifesting as the loss of Achilles tendon reflexes and bowel, bladder, and sexual function.
Neurogenic shock
High cervical injuries can damage the cervical ganglia. These injuries lead to a loss of sympathetic tone, producing neurogenic shock—a state characterized by hypotension and bradycardia.[12]
History and Physical
Patients with an SCI, especially in the setting of acute polytrauma or high spinal segment involvement, may present in shock or cardiorespiratory arrest. A quick primary survey must be performed to assess the airway, breathing, circulation, disability, and exposure. Resuscitative measures should be initiated immediately. A more detailed investigation may be conducted after stabilizing the patient.
Individuals with a suspected spinal cord injury frequently describe various trauma mechanisms, including MVCs, falls, sports injuries, or penetrating trauma. Neurological symptoms commonly reported by these patients include weakness, tingling, or sensory loss in specific areas of the body below the level of injury. Pain, ranging from localized discomfort to radiating pain, may also be reported, depending on the injury's severity and location. Additionally, patients may note motor weakness, manifesting as difficulty moving the limbs or performing previously routine activities. Complaints of urinary or fecal incontinence, difficulty voiding, or loss of bowel control are frequently encountered following an SCI. Respiratory symptoms, such as tachypnea, difficulty coughing, or impaired breathing, may occur due to cervical or thoracic spinal segment involvement.
Chronic SCI may arise from unresolved traumatic cord lesions or etiologies like malignancy and infection. Patients with insidious SCI causes may report persistent back pain that worsens at night or with movement and weight-bearing. Constitutional symptoms may be reported, such as unexplained weight loss, intermittent fever, anorexia, and generalized weakness. The back may feel stiff and have limited mobility. Sensorimotor weakness progresses as the lesion grows. Patients may initially feel paresthesias in the involved nerve's distribution area and develop motor weakness later. Loss of grasp, ambulation, and bladder and bowel function may emerge in late stages. Cardiorespiratory compromise may result from a high spinal injury. Risk factors that may be elicited include chronic smoking, previous cancer treatment, migration from a place where tuberculosis is endemic, recent surgery, and immunosuppression.
On physical examination, clinicians typically observe neurological deficits below the injury level, including decreased motor strength, altered sensation (hypoesthesia or anesthesia), and abnormal reflexes. Some individuals may develop the classic signs of one of the spinal cord syndromes, depending on the lesion's location and severity. Patients with acute trauma may have signs of multiple injuries, such as craniofacial and limb fractures, abrasions, bruises, bleeding, altered sensorium, thoracoabdominal tenderness, hypotension, and respiratory distress.
On the other hand, patients with chronic SCI may exhibit muscle wasting or atrophy in areas affected by paralysis or disuse, alongside signs of spinal deformity such as abnormal curvature, tenderness, or palpable bony abnormalities. Skin changes indicative of pressure ulcers, such as redness, blistering, or breakdown over bony prominences due to immobility, may be noted. The cord injury pattern depends on the lesion's site and extent.
Evaluation
Imaging Studies for SCI Assessment
SCIs most often occur in the context of significant trauma. Thus, a comprehensive clinical assessment for concurrent injuries is necessary at the time of presentation. Recognition of the above injury patterns can help localize the location and type of injury. A thorough clinical examination, including a precise motor and sensory function assessment, is imperative for accurate classification.
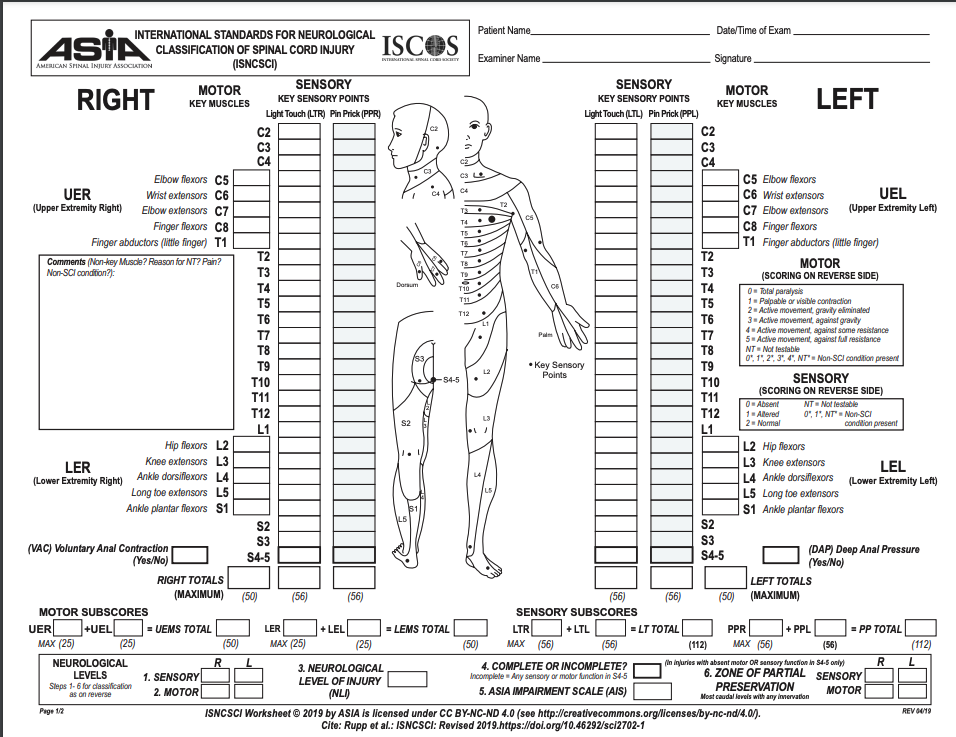
Spinal cord injuries are graded using the American Spinal Injury Association (ASIA) Impairment Scale. The grading system varies based on injury severity from letters A to E (see Image. ASIA Spinal Cord Injury Scoring Sheet).
- ASIA A: Complete injury with loss of motor and sensory function
- ASIA B: Incomplete injury with preserved sensory function but complete loss of motor function
- ASIA C: Incomplete injury with preserved motor function below the injury level. Less than half of these muscles have Medical Research Council (MRC) grade 3 strength.
- ASIA D: Incomplete injury with preserved motor function below the injury level. At least half of these muscles have MRC grade 3 strength.
- ASIA E: Normal motor and sensory examination [13]
Imaging is vital in identifying injuries accurately. Plain radiographs have been used traditionally. However, more sensitive advanced imaging techniques, particularly computerized tomography (CT or CAT scan) and magnetic resonance imaging (MRI), have now become more widely used than plain radiography in assessing spinal injuries. CT identifies fractures with better resolution than x-rays. CT can reveal vertebral fractures and raise suspicion for spinal cord injuries. However, CT has inferior sensitivity for soft tissue injuries.
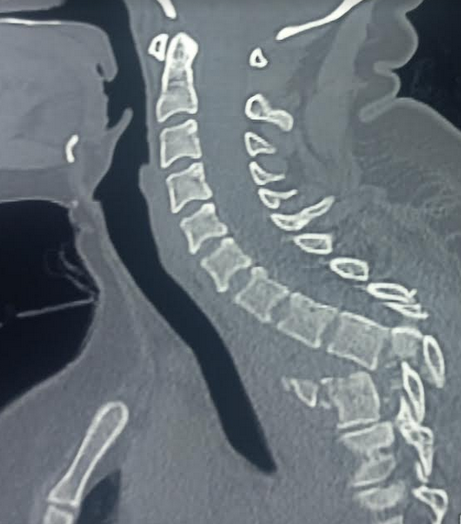
MRI is more reliable than CT when looking for soft tissue pathology, including SCI. This modality can accurately locate the SCI level.[14] MRI can likewise help with prognostication. Several clinical scoring systems can predict SCI prognosis using MRI findings (see Image. Traumatic Thoracic Spondyloptosis on MRI).[15] Early SCI findings on MRI include spinal cord compression, contusion, edema, transection, and hemorrhage, and ligamentum flavum bulging.[16] Subacute findings include spinal cord edema, subacute progressive ascending myelopathy, and syrinx.[17]
Traumatic disc herniation is seen in vertebral disc dislocations and hyperextension injuries. The herniation includes the nucleus pulpous and annulus fibrosus. Associated MRI findings may include epidural hematoma, pseudomeningoceles, extradural fluid collections, craniocervical artery damage (eg, carotid and vertebral artery), and vertebral fractures.[18]
Emerging MRI Modalities
Quantitative MRI techniques, including magnetization transfer, magnetic resonance relaxation mapping, and diffusion imaging, aid in assessing the microstructural neuronal characteristics associated with myelination state and axonal degeneration or regeneration. A diffusion tensor tractography (DTT) reconstructs significant nerve bundles in 3-dimensional environments based on anisotropy features. DTT may be created from diffusion tensor imaging data using a computational technique. This modality can distinguish between damaged and deformed nerve fibers and healthy tissue. DTT provides information on the SCI's extent and may thus be used to plan surgical interventions. Implanting an epidural or intrathecal pressure-monitoring device may help us better understand spinal cord perfusion pressure parameters following an injury.[6]
Treatment / Management
Acute SCI Treatment
Treatment begins at the site of injury. Paramedics and emergency medical services staff are crucial in stabilizing and transferring the patient to an appropriate facility. Immobilization can help prevent complicating existing injuries. Life threats or concurrent traumatic injuries in severe trauma cases must be addressed immediately. Hypotension and shock can aggravate the SCI and reduce the likelihood of neurologic recovery. Immediate measures are necessary to stabilize cardiorespiratory function. Emergent surgical decompression, if warranted and feasible, may lessen the injury's extent.[19][20] This procedure helps stabilize the spine, prevent pain, reduce deformity, and relieve compression from a herniated disc, blood clot, or foreign body.
Maintaining a systolic blood pressure at or greater than 90 mm Hg and a mean arterial pressure around 85 mm Hg to 90 mm Hg has been recommended by the 2002 and 2013 American Association of Neurological Surgeons and Congress of Neurological Surgeons' (AANS/CNS) guidelines. Thus, despite little evidence supporting the practice, a MAP greater than 85 mm Hg is currently targeted as part of SCI management. The most recent AANS/CNS recommendations advise maintaining MAP goals for a week.
Steroid administration is still debatable in SCI treatment. Steroids were initially thought to enhance anti-inflammatory mechanisms, reduce secondary SCI, and increase cell viability. Initial studies suggested potential benefits from steroid administration, yet subsequent research has failed to substantiate any such advantage.
Indications for early intubation include higher SCI levels (above C5), total paralysis, low lung volumes on chest radiographs, and the presence of concomitant injuries, especially chest wall or intrathoracic lesions. Tracheostomy may be advantageous for patients who may require mechanical ventilation longer than 2 weeks following injury.[1]
Patients with acute urinary retention should have a urinary or suprapubic catheter inserted to relieve lower tract discomfort caused by a full bladder. The urethral catheter, which should be 16 to 18 French in size, may be inserted as a first line of treatment. Clean intermittent catheterization results in fewer problems, a higher spontaneous voiding rate, and a lower incidence of urinary tract infections (UTIs). Patients can develop greater tolerance to clean intermittent catheterization if the nursing team focuses on thorough training and precise catheter placement. Additional outpatient support services may be useful. Patients typically welcome education.[21]
The Neurocritical Care Society recommends starting deep vein thrombosis (DVT) prophylaxis as soon as possible and no later than 72 hours after the SCI. The Consortium of Spinal Cord Injury does not define a specific timing but proposes using low-molecular-weight heparin in the acute care period to avoid DVT. Daily bleeding risk assessment must be performed to avoid delays due to bleeding concerns. Enoxaparin is superior to unfractionated heparin in preventing pulmonary embolism in patients with SCIs.
Patients with SCIs are best managed in neurological intensive care units, where personnel are proficient in managing SCIs. Dedicated trauma units must be identified for smooth patient transfer and care transition. Patients have optimum outcomes with intense rehabilitation therapy under physiatrists, physical therapists, and occupational therapists' guidance. Rehabilitation is continued on an outpatient basis after hospital discharge.
Trials involving the administration of nimodipine, gacyclidine, thyrotropin-releasing hormone, riluzole, gangliosides, minocycline, magnesium, and acidic fibroblast growth factor in patients with spinal cord injuries have not shown significant benefits, though further studies are ongoing.[22][23][24] Presently, high-dose steroids remain the cornerstone of acute SCI treatment.
Stem Cell Therapy
Stem cell treatments for spinal cord injuries can be categorized into supportive and loading therapies. Supportive stem cell therapy utilizes nonneural stem cells like bone marrow, umbilical cord, and adipose tissue-derived mesenchymal stem cells (MSCs). These cells are administered intravenously or intrathecally due to limited migration to the target area and differentiation into neural cells. MSCs release neurotrophic factors that can repair the injured area, yet their ability to replenish the nervous system is constrained. Most clinical trials focus on this therapy due to easier MSC preparation and regulatory compliance.
In contrast, loading therapy employs stem cells capable of producing neural cells, such as olfactory ensheathing and neural progenitor and stem cells derived from embryonic stem cells. Engraftment of these cells has a greater likelihood of replacing lost nerve cells in terms of functionality, though the procedure requires invasive transplantation methods and complex cell preparation processes.[25]
Chronic SCI Treatment
Chronic SCI management depends on the underlying etiology. The treatment should address the neurologic deficits and primary disorder. Complications such as pressure ulcers, secondary bacterial infection, and urinary dysfunction must also be addressed. Rehabilitation and supportive care are essential in optimizing quality of life and functional outcomes for people with chronic SCI, regardless of the underlying etiology. Combined modalities and seamless interprofessional collaboration are thus often necessary in treating chronic SCIs.
Differential Diagnosis
The diagnosis of SCIs is based on the patient’s presentation, with most cases emerging from a traumatic event. However, a broader differential must be considered for sensorimotor weakness when the onset and preceding events are unclear. Thus, the following conditions must be included in SCI's differential diagnosis:
- Central Nervous System Pathologies
- Cerebrovascular accident
- Postictal (Todd) paralysis
- Hemiplegic migraine
- Multiple sclerosis
- Peripheral Nerve Pathologies
- Guillain-Barré syndrome
- Transverse myelitis
- Tick paralysis
- Neuromuscular Junction Pathologies
- Myasthenia gravis
- Organophosphate toxicity
- Botulism
- Other Pathologies
- Hypoglycemia
- Hypokalemic periodic paralysis
- Hypocalcemia
- Diabetic neuropathy
- Conversion disorder
A thorough clinical investigation and prudent use of diagnostic examinations can help differentiate SCI from these disorders.
Prognosis
SCI's prognosis is generally poor. No treatment leads to recovery. Less than 1% of patients with SCIs recover complete function before hospital discharge. The degree of disability directly correlates with the injury level, with higher-level injuries resulting in more significant disability and higher complication rates. Patients with acute SCIs have significantly increased mortality in the 1st year following injury, and those that survive have decreased life expectancy. Only 12% go on to hold employment, and less than one-half get married.
Meanwhile, the prognosis of chronic SCI depends on the underlying etiology, injury severity, and timeliness of treatment. Patients who develop various complications due to delayed treatment or have a serious underlying SCI cause generally have a poor prognosis.
Complications
Spinal cord injuries are associated with numerous complications, such as UTIs, pressure sores, DVT, autonomic dysreflexia, and chronic pain. Autonomic dysreflexia occurs in patients with spinal cord injuries at or above T6, often manifesting as orthostatic hypotension. Orthostatic hypotension symptoms are challenging to treat. Symptomatic management with abdominal binders, elastic stockings, peripheral vasoconstrictor medications like midodrine, and mineralocorticoids like fludrocortisone can help. Increased salt intake may also help with volume expansion and symptom control. Significant indirect SCI costs include lost mobility, inability to work, and heavy caregiver burden.[26] The most common causes of mortality from SCIs are pneumonia and sepsis.
Deterrence and Patient Education
Primary preventive measures for SCIs focus on raising awareness of risk factors such as MVCs and falls, promoting safety measures like seatbelt use and helmet-wearing, and advocating for legislation enforcement to uphold safety standards. Environmental modifications, such as installing handrails and improving lighting, further reduce the risk of accidents in public spaces, workplaces, and homes. Prevention centers can help mitigate factors leading to traumatic injuries, like improvement in motor vehicle safety, gun control, and programs aimed at violence prevention. Smoking cessation, occupational safety, and regular health check-ups minimize the risk of chronic SCI.
Secondary prevention strategies include immediate medical attention and proper spine immobilization to prevent SCI exacerbation. Patient education is integral to clinical management. Counseling on prognosis, complications, and outcomes must be provided. Early initiation of rehabilitation programs, such as physical and occupational therapy, supports functional recovery and minimizes disability.
Vigilant monitoring, proactive management of complications like pressure ulcers and UTIs, access to assistive devices, and strong support networks enhance mobility, independence, and overall well-being for individuals with SCI. Support groups aid in addressing social and emotional challenges such as anxiety, frustration, loneliness, and depression, ensuring patients receive comprehensive support and guidance throughout their journey with SCI. By implementing these preventive measures, healthcare professionals and communities can collaboratively work toward reducing SCI incidence and severity, ultimately improving safety and quality of life for those affected.
Pearls and Other Issues
SCIs result from spinal cord damage, leading to motor, sensory, and autonomic dysfunction below the injury level. Prompt diagnosis through neurological assessment and imaging, such as MRI or CT scans, is crucial for accurate classification. Effective management involves acute stabilization to prevent secondary injury, potentially requiring surgical intervention for decompression or stabilization of vertebral fractures.
Following diagnosis, long-term care focuses on rehabilitation to optimize functional recovery and quality of life. This includes physical and occupational therapy, alongside assistive devices, to enhance mobility and independence. Proactive management of complications like pressure ulcers and UTIs is essential to prevent further harm and improve outcomes for patients with SCI. Overall, timely intervention and comprehensive care are vital in mitigating the impact of SCI and promoting better patient outcomes.
Enhancing Healthcare Team Outcomes
Optimizing the care of patients with SCI requires interprofessional collaboration. Evaluation by a neurosurgeon or spine surgeon at the time of injury can help minimize the extent of the initial lesion. Nursing care can prevent catheter-associated UTIs, pressure sores, and aspiration pneumonia. Physical and occupational therapists can help maximize the patient’s level of function. Social workers can coordinate disability services and reimbursements. A psychiatrist should be available to help the patient with depression, which is common following spinal cord injuries. Pain management specialists can help manage chronic pain. Collaborative teamwork enables sharing knowledge, skills, and resources, leading to more accurate diagnoses, effective treatment plans, and improved patient outcomes.[26][27]