Introduction
The heart muscle is the myocardium or middle layer of the heart walls. The myocardium is responsible for the contractile function of the cardiac pump. Composed of cardiomyocytes, the heart muscle has distinctive cellular and physiological features allowing it to generate force to maintain adequate tissue and organ perfusion throughout the entire body. Heart muscle makes up one of the earliest functioning embryonic organs that continues to contract and beat from development throughout a lifetime. Supplied by a complex system of coronary vasculature, cardiac lymphatics, and autonomic innervation, cardiomyocytes line the thickest layer of every chamber of the heart. Cardiovascular diseases are the leading cause of mortality worldwide. A vast number of these diseases involve the heart muscles with diverse mechanisms of pathophysiology, leading to contractile dysfunction, cell damage and death, and cardiac pump failure. However, numerous interventions, treatment options, and therapies are aimed at minimizing damage, restoring functionality, preventing the occurrence, and reducing the risk of cardiovascular disease.
Structure and Function
Three distinct layers comprise the heart walls, from inner to outer:
- Endocardium
- Myocardium
- Epicardium (inner layer of the pericardium)
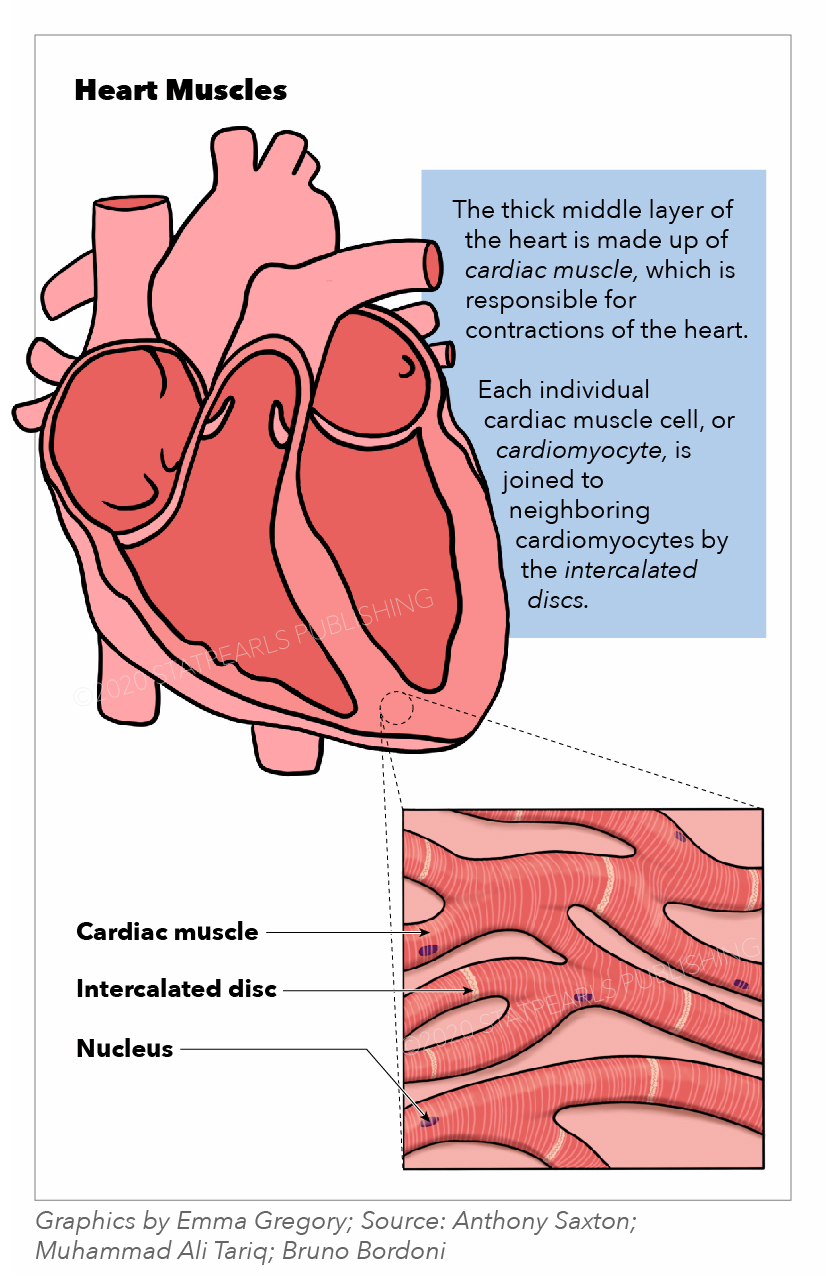
The muscles of the heart, termed the myocardium, make up the middle and thickest layer of the heart wall. This layer lies between the single-cell endocardium layer, which lines the inner chambers, and the outer epicardium, which makes up part of the pericardium that surrounds and protects the heart. Histologically, heart muscles are composed of cells called cardiomyocytes that have unique structures and properties correlating to their contractile function.[1] Cardiomyocytes are striated, uninucleate muscle cells found exclusively in the heart muscle. A unique cellular and physiological feature of cardiomyocytes are intercalated discs, which contain cell adhesions such as gap junctions, to facilitate cell-cell communication. These discs reduce internal resistance and allow action potentials to spread quickly throughout the entire heart muscle via the passage of charged ions. Thus, the heart muscle acts as a functional syncytium with rapid synchronized contractions that are responsible for pumping blood throughout the body. Functionally, the heart muscles rely on electrochemical gradients and the potential to generate contractile force for each heartbeat.
The sinus node, located within the right atrial myocardium, spontaneously depolarizes and thus determines the heart rate. These depolarizations are currents of ion influx that are carried from the sinus node to the heart muscle via conducting cells. When the depolarization reaches the heart muscle, voltage-gated sodium channels open, allowing a rapid influx of sodium ions into the cardiomyocytes, depolarizing the cells. The positive membrane potential triggers voltage-gated potassium and then calcium channels to open, allowing potassium to rush out and calcium to rush in. The initial influx of calcium is necessary for the second release of calcium from the sarcoplasmic reticulum found within the heart muscle cells. The accumulation of intracellular calcium ions binds to troponin C, moving tropomyosin aside to allow actin-myosin binding and cross-bridge cycling responsible for muscle contraction.[1] The amount of calcium released is directly proportional to the amount of actin-myosin interaction allowed and thus correlates with the contractile force of the heart muscle generated. Physiologically, this corresponds with parameters such as stroke volume, ejection fraction, and cardiac output used to assess heart function. At the end of each cycle, calcium gets restored to the sarcoplasmic reticulum via SERCA (Sarco(endo)plasmic reticulum (SER) Ca2+ ATPase) pumps, while sodium-potassium and sodium-calcium ATPase pumps restore the cardiomyocyte membrane potential so the cycle can repeat with the next incoming depolarization.[1]
Embryology
The heart muscle originates from the mesoderm layer and begins forming during the third week of embryonic development. The mesoderm serves as the primary source for myocardial precursor cells, which make up the cardiogenic or primary heart field during early development. A primitive, horseshoe-shaped endothelial heart tube is formed and begins contracting to facilitate the embryo’s early circulation system. Within the next several weeks, the proliferation of cardiomyocytes is necessary for expanding the myocardial layer and generating the multichambered system of the mature heart.[2] While existing cardiomyocytes contribute to the growth of the myocardium via proliferation and organization, new heart muscle cells are also recruited from adjacent mesenchymal layers that further expand the muscle layer.[2] Following myocardial development, the heart walls undergo further maturation, compaction, and trabeculation. Dilatations or swellings of the heart tube embryonic structures, along with neural crest cell migration, facilitate the development of the chambers and inflow/outflow tracts. These processes result in a mature and fully functional, contracting heart by the eighth embryonic week and throughout adulthood.
Blood Supply and Lymphatics
The heart muscles’ blood supply comes directly from the system of coronary arteries that runs within the epicardial layer. Two main coronary arteries, the left coronary artery (LCA) and the right coronary artery (RCA), branch directly off the aorta via the coronary Ostia. These arteries and their branches supply tributary arteries that run perpendicular to the heart surface and transverse from the epicardium, through the myocardium, and down to the endocardium.[3] The LCA quickly branches into the left anterior descending (LAD) coronary artery and the left circumflex (LCX) coronary artery. The LAD runs vertically down the interventricular groove towards the apex and supplies blood to the anterior left ventricular myocardium, the anterior two-thirds of the interventricular septal myocardium, and the anterolateral papillary muscle connecting the mitral valves. The LCX courses horizontally along the atrioventricular groove and gives rise to the left obtuse marginal coronary artery, together supplying the lateral and posterior left ventricular myocardium. The RCA runs horizontally along the right atrioventricular groove and gives rise to the right acute marginal coronary artery, which supplies the right ventricular myocardium. The RCA also gives rise to the posterior descending artery (PDA) in about 90% of the human population (the PDA comes from the LCX in the other approximately 10%), which supplies the posterior myocardium of both ventricles, the posterior one-third of the interventricular septal myocardium, and the posteromedial papillary muscle of the mitral valves.[3] Blood flow via the coronary arteries to the myocardium occurs during diastole and ventricular relaxation via the passive flow of blood into the aortic Ostia. During systole and ventricular contraction, the coronary arteries become compressed and thus impede myocardial blood flow.
The venous system of the heart muscles runs parallel to the coronary arteries. Venous drainage of the left ventricular myocardium is completed by the interventricular vein and the great cardiac vein, which drains into the coronary sinus, found in the posterior right atrioventricular groove, which then drains into the right atrium.[3] The anterior cardiac veins are responsible for draining blood from the right ventricular myocardium directly into the right atrium.[3]
The cardiac lymphatic drainage system is comprised of lymphatic capillaries and pre-collector vessels organized in plexuses within each of the heart wall layers.[4] These lymphatic vessels and plexuses flow from the subendocardium, through the myocardium, up through the subepicardium, into the mediastinal lymph nodes, and ultimately drain into both left and right venous angles between the internal jugular veins and the subclavian veins. The source of flow for lymphatic drainage comes from contractions of the myocardium, which generate force to propel fluid movement through the system to the lymph nodes.
Nerves
Heart muscles are innervated primarily by two nerves, the accelerans nerve and the vagus nerve, which provide sympathetic and parasympathetic stimulation from the autonomic nervous system, respectively. Intrinsic ganglia for the myocardium are present in the epicardium, which receives signals from post-ganglionic sympathetic connections coming from the accelerans nerve and pre-ganglionic parasympathetic connections from the vagus nerve.[5] Most post-ganglionic sympathetic connections synapse directly with the heart muscle cells, releasing norepinephrine as the primary neurotransmitter.[5] Upon binding, norepinephrine stimulates beta-adrenergic receptors to increase contractility of the myocardium via increasing calcium influx. Preganglionic parasympathetic fibers synapse first with the epicardial intrinsic ganglia, and then post-ganglionic neurons directly synapse with the myocardium.[5] Acetylcholine is the primary neurotransmitter for myocardial parasympathetic signals, acting on muscarinic (M2) receptors on the cardiomyocytes.
Muscles
The muscle layer of the heart is termed the myocardium and is made up of cardiomyocytes. The myocardium is found in the walls of all four chambers of the heart, though it is thicker in the ventricles and thinner in the atria. This disparity is due to the difference in the generation of the force of contraction needed for propelling blood between the atria and the ventricles, with ventricles requiring much more power.
Physiologic Variants
Chronic primary hypertension is a common and prevalent disease affecting a large percentage of the United States population. Over long periods, chronic systemic hypertension can result in structural and functional changes to the heart muscle wall. Due to chronic increases in afterload, the pressure in which the left ventricular muscle must contract against increased mean arterial pressure, and the heart muscle responds with compensatory cardiomyocyte hypertrophy. The ventricular muscle wall thickens to reduce wall stress with resulting increase in the wall thickness to the chamber diameter ratio.[6] Left ventricular hypertrophy (LVH) thus characteristically demonstrates a geometry of concentric thickness and is a physiological response to and a common complication of even mild chronic hypertension.[6] Physical examination findings of LVH may include an enlarged point of maximal impulse (PMI) and an S4 gallop upon cardiac apex auscultation.
Normal aging processes alter heart muscle structurally and physiologically. Arteries become less compliant and stiffen over time, and in older years, this results in an increase in afterload due to greater pressure against which the heart muscle must contract. As a compensatory response, left ventricular thickness increases from cardiomyocyte hypertrophy. Over time, cardiomyocytes diminish with age from apoptosis, necrosis, or autophagy leading to an overall decrease in cardiomyocyte number in aged heart muscle.[7] As a compensatory mechanism, the remaining cardiomyocytes may hypertrophy or undergo pathological remodeling. These changes result in a decrease in cardiac compliance and an increase in wall stiffness. Within the heart muscle cells, age-related changes induce a shift from the alpha-myosin heavy chain to the beta-myosin heavy chain with reduced cross-bridge cycling activity.[7] This condition ultimately leads to contractile decline and diastolic dysfunction in the aging heart muscle. Calcium homeostasis is also affected during aging processes due to a reduced ability of SERCA pumps and the sodium-calcium pumps to effectively restore resting membrane potential calcium levels.[7] Disrupted calcium homeostasis affects heart muscle relaxation mechanics and thus leads to diastolic dysfunction.
Surgical Considerations
A significant yet underrecognized heart muscle complication after non-cardiac surgery is perioperative myocardial injury (PMI), which is distinct from myocardial infarction. Risk factors for PMI before and after surgery include an age greater than or equal to 65 years and a preexisting history of atherosclerotic disease. PMI is an acute increase in high-sensitivity cardiac troponin T (hs-cTn) plasma concentrations.[8] PMI often presents without chest pain, dyspnea, or other typical cardiac injury symptoms and is, therefore, routinely missed during clinical workups perioperatively. However, PMI correlates with a significantly increased risk of 30-day mortality post-noncardiac surgery.[8] To effectively diagnose PMI, hs-cTn screening should be used perioperatively to detect and quantify cardiomyocyte injury to reduce the risk of short and long-term mortality.
Clinical Significance
Coronary artery disease (CAD), also termed ischemic heart disease, is the most prevalent cardiovascular disease and the leading cause of global mortality, with significant implications and consequences for heart muscle functionality. CAD is characterized by the formation of atherosclerotic plaques within the coronary arteries resulting in a decrease in blood flow and oxygen and nutrient delivery to the myocardium. Manifestations of CAD are termed acute coronary syndromes (ACS) and include stable angina, unstable angina, and others from the supply-demand mismatch of insufficient oxygen perfusion from at least 70% occlusion of the myocardial vascular supply. The most severe manifestations of CAD are myocardial infarctions, colloquially termed “heart attacks.” Myocardial infarctions (MIs) divide into ST-segment elevation myocardial infarctions (STEMI) and non-ST-segment elevation myocardial infarction (NSTEMI), depending on total or near-total coronary vascular occlusions, respectively, and subsequent findings on electrocardiogram (EKG).
The location of myocardial infarctions within the heart can be generally localized via which specific leads are affected with abnormal ST-segment and/or T-wave morphologies on an EKG. MIs demonstrate cardiomyocyte necrosis, and diagnostic findings include positive cardiac troponin (cTn) tests and creatinine kinase myocardial band (CK-MB) tests.[9] Following MIs, the affected heart muscle wall is often weaker than it was before the MI from cell injury and death, inflammation, and fibrotic replacement that decreases heart muscle contractile function, which increases the risk for heart failure, free wall ruptures, arrhythmias, sudden cardiac death, and other complications. Treatment for MIs includes pharmacotherapy such as antiplatelet drugs and fibrinolysis, interventions such as percutaneous coronary interventions (PCIs), or surgeries such as coronary artery bypass grafting (CABG) aimed at restoring perfusion and vascularization of the myocardium.[9]
Cardiomyopathies are inherited or acquired diseases of the heart muscle that affect structure and functionality in the absence of other cardiovascular disease processes, such as CAD. Hypertrophic cardiomyopathy (HCM) is the most common genetically inherited heart disease and follows an autosomal dominant inheritance pattern. The pathophysiology of HCM comes from mutations in myocardial sarcomere genes leading to myofibrillar disarray, severe thickening, hypertrophy of the heart muscle wall, and diastolic dysfunction. Manifestations of HCM include dyspnea, syncope, palpitations, and sudden death, and most commonly presents in adolescence. Dilated cardiomyopathy (DCM) is characterized by eccentric hypertrophy, dilation of the cardiac muscle wall, and systolic dysfunction.[10] DCM is the final myocardial response to a variety of genetic and environmental stresses such as chronic alcohol abuse, viral or parasitic infections, certain chemotherapies, and other etiologies, with the most common being idiopathic DCM.[11] Treatment for cardiomyopathies includes pharmacotherapy to reduce heart muscle demand, such as calcium-channel blockers or beta-blockers, implantable cardioverter-defibrillator (ICD) placements to prevent life-threatening arrhythmias, or heart transplantation.
Myocarditis is the term for inflammation of the heart muscle and has several etiologies. The most common etiology in the Western world is viral infections (infectious myocarditis), but other causes include toxic reactions or allergies to drugs, autoimmune diseases, or other infections (bacterial, fungal, parasitic). Myocarditis involves damage and loss of both cardiomyocytes and cardiac vascular endothelial cells, both of which are common targets of infection from inflammatory processes and white blood cell (often lymphocytic) infiltration into the heart muscle wall.[12] Complications include interstitial cardiac fibrosis, wall motion abnormalities, arrhythmias, heart failure, myocardial infarctions, reduced ejection fraction, and sudden cardiac death.[12] Myocarditis has a diverse presentation with non-specific symptoms such as chest pain, dyspnea, and flu-like manifestations, but can also be symptomless.
Heart failure (HF) is the common end-stage pathway and clinical manifestation of cardiac pump dysfunction from a variety of etiologies, including many diseases affecting the heart muscle. HF can be categorized into different groups, such as acute vs. chronic, right heart vs. left heart, and systolic (reduced ejection fraction) vs. diastolic (preserved ejection fraction), each presenting with distinct clinical characteristics. HF is essentially defined as the inability of the heart to pump adequately, leading to congestion, reduced organ perfusion, and functional impairment.[1] Etiologies and risk factors for HF are diverse and encompass processes such as myocardial injury or infarction, CAD, chronic hypertension, valvular dysfunction, arrhythmias, cardiomyopathies, and numerous other pathways. The pathophysiology resulting in HF involves complex interactions of systems such as neurohormonal activation, peripheral vascular effects, and physiological processes within the heart muscle itself.[1] Treatment for HF involves pharmacotherapy such as ACE inhibitors and beta-blockers to decrease heart stress and workload or inotropes to increase contractility. Therapies for advanced heart failure include mechanical circulatory support such as ventricular assist devices (VADs) or heart transplantation.