Continuing Education Activity
Heterotopic ossification is frequently observed in the rehabilitation population. It consists of the formation of mature, lamellar bone in extraskeletal soft tissue where bone does not usually exist. Patient populations at risk of developing heterotopic ossification include patients with burns, strokes, spinal cord injuries, traumatic amputations, joint replacements, and traumatic brain injuries. This activity describes the pathophysiology, evaluation, and management of heterotopic ossification and highlights the role of the interprofessional team in its management.
Objectives:
- Identify the etiology of heterotopic ossification.
- Describe the presentation of a patient with heterotopic ossification.
- Review the management options available for heterotopic ossification.
- Explain the importance of enhancing coordination amongst interprofessional team members to improve outcomes for patients with heterotopic ossification.
Introduction
Heterotopic ossification (HO) is a frequent complication in the rehabilitation setting which consists of the formation of mature, lamellar bone in the extraskeletal soft tissue where bone does not usually exist. Patient populations at risk of developing HO are those with burns, stroke, spinal cord injury (SCI), traumatic amputation, joint replacement, and traumatic brain injury (TBI). There are other genetic causes of HO such as fibrodysplasia ossificans progressiva, Albright’s hereditary osteodystrophy and progressive osseous heteroplasia that are not related to rehabilitation complications and will not be discussed here.
Etiology
There are 2 main categories of HO: traumatic (fracture, arthroplasty, muscular trauma, joint dislocation, burns) versus neurogenic (stroke, SCI, TBI, brain tumors) [1]. In total joint arthroplasty, HO most commonly occurs for the replacement of hips, knees, elbow, and shoulder. Chronic muscular trauma leads to what is traditionally known specifically as traumatic myositis ossificans. The most common sites for traumatic myositis ossificans are the quadriceps femoris muscle and the brachialis muscle. The most common sites for neurogenic HO are the hips, elbows (extensor side), shoulders, and knees. Uncommon sites of HO that may be encountered in a rehabilitation setting are incisions, kidneys, uterus, corpora cavernosum, and the gastrointestinal (GI) tract. The exact cause and mechanism of neurogenic HO are unknown. More will be discussed in the Pathophysiology section. Risk factors for developing HO are spasticity, older age, pressure ulcer, the presence of deep vein thrombosis (DVT), having a tracheostomy, long bone fractures, prior injury to the same area, edema, immobility, long-term coma, and severity of injury (trauma, TBI, SCI, stroke). High/moderate risk factors in the THA population include men with bilateral THA, prior history of HO, ankylosing spondylitis, diffuse idiopathic hyperostosis, or Paget's disease. [2]
Epidemiology
In the total hip arthroplasty population, the incidence varies. In those with high-risk factors, incidence can be as high as 90%. Overall rates for developing HO in the THA population is closer to 53%. HO is twice as common in males versus females, but it is noted that females older than 65 years old have an increased risk of developing HO. The incidence of neurogenic HO is 10% to 20%. When differentiating between the incidence of HO in adult SCI patients is 20% to 30% and in adult TBI patients is 10% to 20% [1]. HO in the pediatric TBI population ranges from 3% to 20%. [3]
Pathophysiology
The exact mechanism of HO in traumatic and neurogenic HO is unknown [4], but 2 common factors precede the formation of HO, the first being trauma or an inciting neurological event. In the SCI population, it is hypothesized that acute rehabilitation, transfer activities, and repeated microtrauma can add up during activities of daily living (ADL) can cause an accumulation of mechanical stress that predisposes one to the formation of HO. Second, after trauma or neurological injury, is the tissue expression of bone morphogenic proteins (BMPs). BMPs stimulate mesenchymal spindle stem cells, also known as satellite cells, to migrate to the injured area and transform into fibroblasts and eventually, osteoblasts. It has been proposed that alkaline phosphatase also plays a role in ectopic hone formation. Alkaline phosphatase acts to suppress inhibitors of bone formation is known to be elevated in vascular smooth muscle tissue in the presence of inflammatory cytokines and macrophages [5]. After migration, mesenchymal spindle cells begin to differentiate into fibroblasts which then start to secrete immature connective tissue composed of collagen and extracellular matrix. With continued tissue irritation, fibroblastic metaplasia is activated, transforming fibroblasts into chondrocytes in a similar process to endochondral ossification. Some of the chondrocytes continue to deposit collagen into the cartilage matrix while the remaining chondrocytes transform into osteoblasts. By 1 to 2 weeks, new osteoid is present within the tissue, and new bone formation starts to form within the osteoid. Osteoblasts then begin to degrade and replace the cartilage with bone. Calcium pyrophosphate within the osteoid is slowly replaced with hydroxyapatite crystals as the bone mineralizes and and starts to mature. [6]
Histopathology
Microscopic examination of biopsy in myositis ossificans revealed an outer zone of hypercellular spindle cells and woven bone surrounding an inner zone of trapped muscle with normal osteoblasts continuing to lay down bone. To distinguish HO from osteosarcoma, HO is zonation from central immature fibrous tissue to peripheral mature bone is characteristic of HO. This helps distinguish HO from osteosarcoma, in which the central zone consists of mature bone [1]. At initial injury, there will be a proliferation of hypercellular spindle cells. Cartilage and woven bone start to form 2 weeks after injury. Trabecular bone starts to form from weeks 2 to 5 with mature fatty bone marrow. After 6 weeks lamellar bone will mature. [7]
History and Physical
HO usually occurs 3 to 12 weeks after inciting injury [8], but can take up to 6 months to present. Look for a recent history of arthroplasty (total hip arthroplasty [THA], total knee arthroplasty), stroke, SCI, TBI, or burn. The most common presentation will be pain and decreased range of motion (ROM). Patients often complain of stiffness in their joints. Other common signs to look for are local edema, effusion, erythema, warmth, and tenderness [1] in the tissue or joint. Localized soft tissue swelling may present like a deep vein thrombosis (DVT). The patient may also present with a low-grade fever. Since spasticity is a risk factor, the patient may present with spasticity near the affected joint. Other risk factors to look for in the history are prolonged coma, tracheostomy or gastric tube, immobility, pressure ulcers and associated long bone fracture. [9] The greatest risk for developing HO also occurs at the 3 to 4 month period post-injury.
Evaluation
Laboratory Studies
Alkaline phosphatase is historically the most commonly ordered lab, but may not be elevated early on in HO formation [10]. It may take up to 2 weeks to be elevated and can rise to 3.5 times the normal value 10-weeks post-injury. Serum alkaline phosphatase greater than 250 has been shown to correlate with HO in the THA population, but there isn't a correlation between the level of alkaline phosphatase and the severity of the injury. Alkaline phosphatase can also be falsely elevated with associated long-bone injuries.
Erythrocyte sedimentation rate (ESR) is another inflammatory marker that is used. ESR greater than 35 mm/hr can indicate the development of HO [11]. C-reactive protein is another inflammatory marker that can be elevated in early HO. Both are non-specific. Creatine kinase (CK) can be used to determine the severity of HO but is not a very specific test. [1] However, one study of 18 spinal cord injured patients determined that an elevated CK may be associated with a more aggressive course of HO and show possible resistance to etidronate therapy. [12]
Imaging
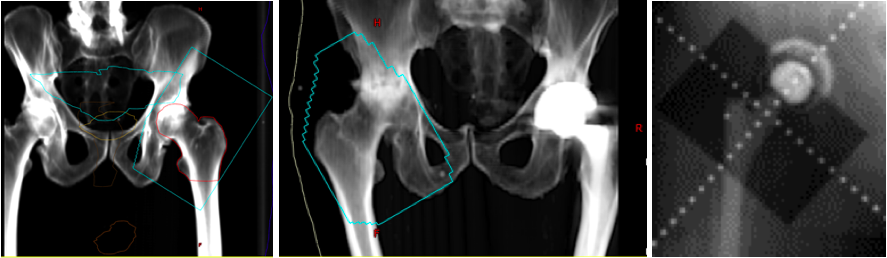
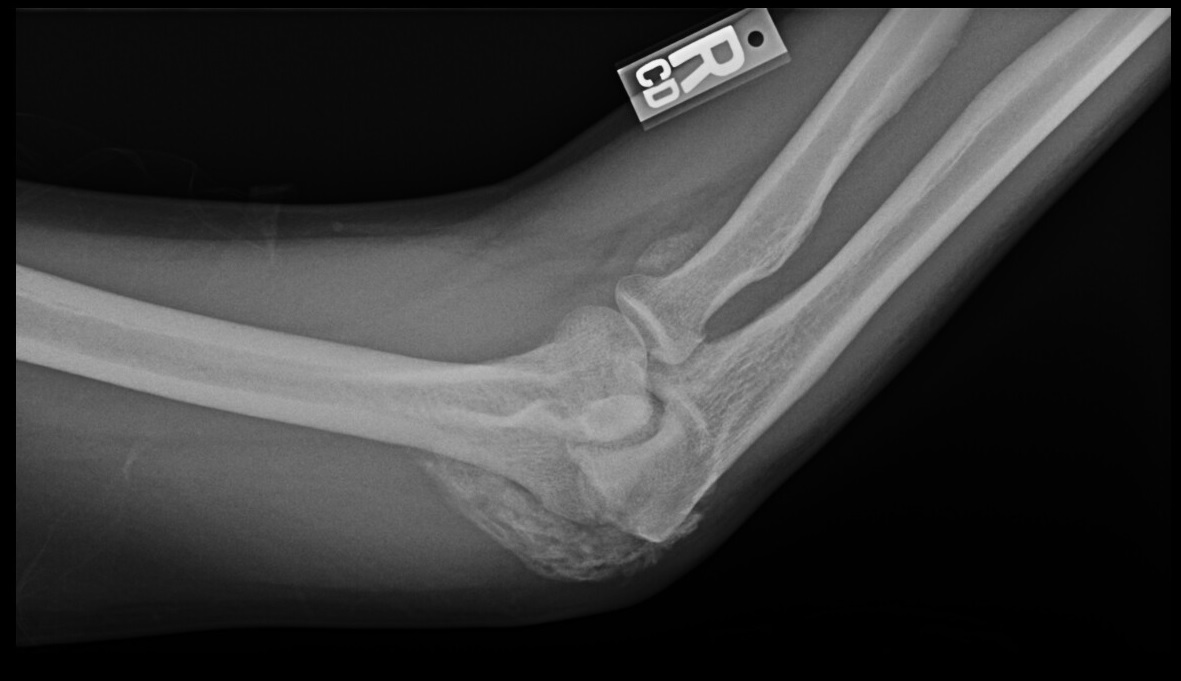
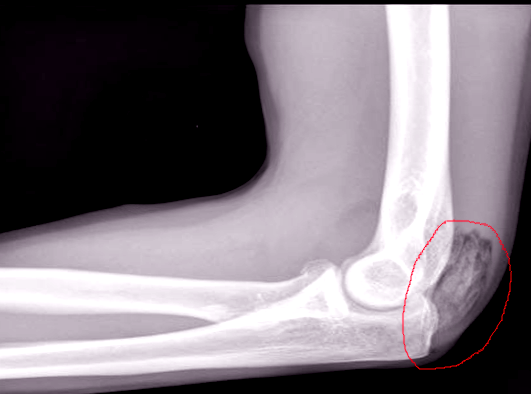
Plain film radiographs show circumferential bone formation around or near a joint with a radiolucent center (figure 1). X-ray is specific for HO but not sensitive in early disease. Plain films may not be positive until 3 to 4 weeks after HO appears on bone scan. Therefore the triple phase bone scan is the most sensitive. A bone scan can reveal HO as early as 2.5 weeks after injury [1].
CT may be used to delineate the area of bone formation in preparation for surgical, but their role in evaluation/diagnosis of HO is not established [5]. MRI may also be used but is not cost effective unless bone encompasses neurologic structures. Other imaging techniques uncommonly used are an ultrasound and 3-dimensional stereolithography.
Treatment / Management
Prophylaxis
The approach to prophylaxis involves identifying patients with high risk of developing HO. Routine prophylaxis is not recommended. Current recommendations for prevention of HO are gentle ROM exercises, indomethacin, etidronate, and external beam radiation, which is primarily used after joint arthroplasty. It is also important to manage risk factors, such as spasticity, in the prevention of HO. Indomethacin is the most commonly used NSAIDs used for prophylaxis [13]. Others NSAIDs that have been proven effective are meloxicam, celecoxib, rofecoxib, and ibuprofen. The recommended dose of indomethacin for prophylaxis in the THA population is 75 to 100 mg per day for 7 to 14 days post-operatively. In the SCI population, 75 mg per day of indomethacin for 3 weeks is the recommended [14]. Careful monitoring must be done for risk of bleeding, especially with concurrent chemoprophylaxis for venous thromboembolism. It is advised to use concurrent prophylaxis for gastrointestinal (GI) ulcers. Twenty mg per day of a selective COX-2 inhibitor can be used with fewer GI side effects compared to traditional NSAIDs [14].
Etidronate is a bisphosphonate that has been approved for prevention of heterotopic ossification in spinal cord injuries and complication of total hip arthroplasty. For spinal cord injury associated HO, the recommended treatment is 20 mg/kg per day for 2 weeks then 10 mg/kg per day for 10 weeks for a total treatment period of 12 weeks. For total hip arthroplasty, recommended treatment is 20 mg/kg per day for 1 month preoperatively and then the same dosing for 3 months postoperatively [14].
External beam radiation is most commonly used after total joint arthroplasty. A single dose of 700 to 800 g (centigray) is administered up to 24 hours preoperatively or within 72 hours postoperatively [15].
Treatment
Current treatment recommendations consist of mobilization with ROM exercises, indomethacin, etidronate, and surgical resection. [5] Early treatment with a passive range of motion exercises should be implemented once the presence of HO is confirmed to prevent ankylosing of joints. Absolute treatment consists of surgical resection of mature bone once the HO has fully matured. This can be 12 to 18 months after the initial presentation [16]. Surgical consultation with an orthopedic surgeon is warranted only if there will be an improvement in function as demonstrated by mobility, transfers, hygiene, and ADLs.
Indomethacin and etidronate are also used to help arrest bone formation in HO, but efficacy in the traumatic brain injury population has not been clearly proven. The most effective treatment option in the TBI population is surgical resection. In the SCI population, the most effective NSAID treatment regiments are either Rofecoxib 25 mg per day for 4 weeks or indomethacin 75 mg daily for 3 weeks. [14]
Differential Diagnosis
In the spinal cord injury population, DVT, cellulitis, abscess hematoma, and tumor (osteosarcoma, osteochondroma) are the most common differential diagnoses that should be ruled out, especially if plain film x-ray is negative. Other common differential diagnoses are hardware infection, thrombophlebitis, and osteomyelitis [1]. A venous Doppler should be ordered to quickly and easily rule out a DVT. Cellulitis and abscess can be difficult to assess because white blood cell count (WBC) and inflammatory markers can be elevated in HO. Elevated alkaline phosphatase can help distinguish HO from other infectious conditions. CT scan/MRI with contrast can help differentiate HO from a hematoma, thrombophlebitis, osteomyelitis, and a tumor but is not the most cost-effective. Triple-phase bone scan remains the most sensitive for detection of HO, especially early on since it can take up to 6 weeks from the initial presentation of symptoms for HO to appear on an x-ray.
Staging
Staging is most often used for surgical planning and is not used much in rehabilitation management. Brooker Classification System, used for severity of HO in the hip, is the most commonly used staging system [8], which uses plain film radiograph to evaluate the presence and size of bone deposition visually. A simplified version has been proposed called the "Della Valle" classification system [17]. None of the most commonly used rating systems take into account patient function or ROM, so a modified Brooker classification scale has been proposed that take these factors into account.
Brooker classification divides the extent of HO formation in the hip into 4 classes:
- Class 1: Islands of bone within the soft tissues around the hip
- Class 2: Bone spurs that originate from the pelvis or proximal end of the femur, leaving at least 1 cm between opposing bone surfaces
- Class 3: Bone spurs originating from the pelvis or proximal end of the femur, reduced space between opposing bone surfaces to less than 1 cm
- Class 4: Ankylosis of the hip
The "Della Valle" classification system [17]:
- Grade A: Absence of HO (or if bone is present, it may be greater than or equal to 1 island of bone of less than 1 cm in length
- Grade B: Presence of greater than or equal to 1 islands of bone at least 1 cm in length with 1 cm distance between opposing surfaces of bone
- Grade C: Bone spurs arising from the pelvis or femur with less than 1 cm between opposing surfaces or bone ankylosis
Prognosis
Complications of HO present itself through decreased function and mobility, peripheral nerve entrapment, and pressure ulcers. Up to 70% of cases involving HA are asymptomatic. Ankylosis, vascular compression, and lymphedema can also be complications manifested in HO [16]. Prognosis is generally good after surgery. Mean time from injury to surgery is 3.6 years. Once the surgery is performed, studies have shown that average ROM in the hip can improve from 24.3 to[5]. After surgery, improvement was maintained in follow up 6 months after surgery. Complications from surgical resection of HO, such as infection, severe hematoma, and DVT [5].
Consultations
General or orthopedic surgery should be consulted once the bone has matured and if HO is causing limitations in patients function, mobility, ability to provide self-care and perform ADLs or if the caretaker has a hard time performing ADLs, including hygiene and daily self-care.
Pearls and Other Issues
- HO is a common rehabilitation complication after joint arthroplasty, TBI, stroke, SCI, and burns.
- Prevention should be a priority in those with a high amount of identifiable risk factors.
- Risk factors include spasticity, older age, pressure ulcer, the presence of DVT, having a tracheostomy, long bone fractures, prior injury to the same area, edema, immobility, long-term coma and severity of the injury.
- Evaluation: Triple-phase bone scan is most sensitive. Plain film radiograph is specific, but may not show up until the bone has matured in up to 6 weeks.
- Prevention with ROM, control of spasticity, NSAIDs (indomethacin, COX-2 inhibitors), bisphosphonates (etidronate) and external beam radiation in joint replacement.
- Treatment with ROM, NSAIDs (SCI population), Bisphosphonates (SCI and THA), and surgery (TBI population)
- Absolute treatment with surgery only after HO has fully matured, which can take up to 12 to 18 months.
- Current pharmacological treatment options are limited, unsafe, and only prevent the progression of the disease. The bone that has already formed can already limit a person's function and still need surgical revision despite ROM exercises and pharmacological intervention. There is limited research on effective options for prophylaxis/treatment, when to start treatment, and continued research on the effectiveness of novel treatments in specific patient populations.
Enhancing Healthcare Team Outcomes
HO is best managed by an interprofessional team that includes orthopedic nurses. The condition is not only difficult to diagnose because of lack of specific markers but its treatment is not satisfactory. Current treatment recommendations consist of mobilization with ROM exercises, indomethacin, etidronate, and surgical resection. Early treatment with a passive range of motion exercises should be implemented once the presence of HO is confirmed to prevent ankylosing of joints. Absolute treatment consists of surgical resection of mature bone once the HO has fully matured. Surgical consultation with an orthopedic surgeon is warranted only if there will be an improvement in function as demonstrated by mobility, transfers, hygiene, and ADLs.