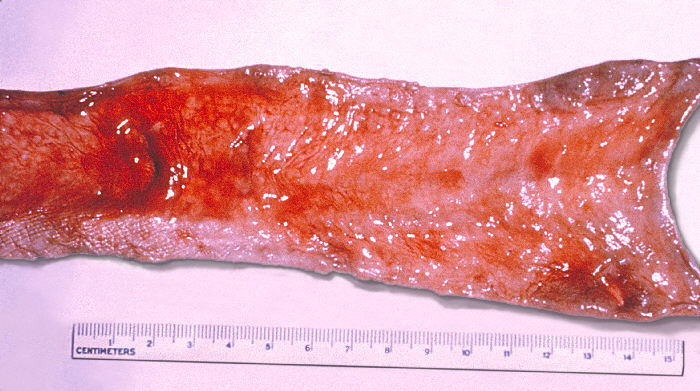
Continuing Education Activity
Typhoid fever is an enteric fever characterized by systemic illness along with abdominal pain and fever in a “step-ladder” pattern. The organism responsible for enteric fever is Salmonella typhi. Other serotypes, Salmonella paratyphi (A, B, C), also cause similar syndromes but with less clinically significant disease. This activity reviews the evaluation and management of typhoid fever and highlights the role of the interprofessional team in improving care for patients with this condition.
Objectives:
- Describe the etiology and epidemiology of typhoid fever.
- Review the appropriate history, physical, and evaluation of typhoid fever.
- Outline the treatment and management options available for typhoid fever.
- Identify interprofessional team strategies for improving care coordination and communication to advance the care for typhoid fever and to improve outcomes.
Introduction
Typhoid fever is also called enteric fever. It is a prospectively, multisystemic illness that has been a public health problem, especially in the developing world. It is caused by Salmonella typhi and Salmonella paratyphi.[1] Enteric fever is a cumulative term that illustrates both typhoid and paratyphoid fever. Paratyphoid is clinically indistinct from typhoid fever; thus, enteric and typhoid fever are used mutually. Typhoid fever is one of the major causes of mortality and morbidity in overcrowded and unhygienic areas though comprehensive research and public health interventions have decreased the occurrence. The disease course ranges from early gastrointestinal distress to nonspecific systemic illness but ultimately may lead to multiple complications. Salmonella is said to spread by the 'four Fs" (flies, fingers, feces, fomites). Fever characteristically comes in a step-wise pattern (i.e., rises and falls alternatively) followed by headache and abdominal pain.
Etiology
The main causative agent of typhoid fever is Salmonella typhi and Salmonella paratyphi, both are members of the Enterobacteriaceae family. Salmonella is a genus[2] that has two species Salmonella enterica serovar and enteritidis classified through extensive analysis by multiplex quantitative polymerase chain reaction (PCR).[3] Both Salmonella typhi and Salmonella paratyphi (A, B, C) are Salmonella enterica serotypes. Nontyphoidal salmonella (NTS) is more typical in children and is mostly limited to gastroenteritis.
Salmonella is transmitted by the fecal-oral route through contaminated water, undercooked foods, fomites of infected patients, and is more common in areas with overcrowding, social chaos, and poor sanitation. It is only transmitted from an infected person to another person, as humans are its only host. Major sources of salmonella are poultry, eggs, and rarely turtles. In one study done on the distribution of salmonella isolates by whole-genome sequencing in chicken slaughterhouses in China, 57% of samples were positive.[4]
Normal flora of the gut is protective against the infection. The use of antibiotics such as streptomycin destroys the normal flora, which heightens its invasion. Malnutrition decreases normal gut flora and thus increases the susceptibility to this infection as well.[5] Hence, the use of broadspectrum antibiotics and poor nutrition amplify the incidence of typhoid fever.
Epidemiology
While the United States reports only about 350 culture-confirmed cases of typhoid fever and fewer than 100 paratyphi A cases each year since 2008, enteric fever remains an important cause of illness worldwide. Approximately 215,000 deaths result from over 26 million cases of typhoid fever and 5 million cases of paratyphoid infection each year worldwide.[6] The incidence of typhoid is more common in low and middle-income countries of south-central Asia and southern Africa than in developed countries. Most cases in developed countries are carried by travelers returning from endemic areas and travelers visiting relatives and friends that are at heightened risk due to their likelihood to be less cautious with sources of food and water. Those less likely to seek vaccination and pretravel consultation are also at heightened risk. Typhoid fever is more prevalent in temperate and tropical climates. It is directly associated with sanitation, sewage, and water treatment system. Salmonella typhi is more common than Salmonella paratyphi, and Salmonella paratyphi A is more prevalent than Salmonella paratyphi B infections. The number of new cases of typhoid fever has been increasing worldwide due to rapid increases in population, pollution, and shortages of pure drinking water. Still, death rates have decreased due to extensive research, changes in treatment modalities, and the invention of new drugs despite growing multidrug-resistance. In the era of routine antibiotics, classic presentations are not always seen. In the United States, splenomegaly and rose spots may be seen in only 10% and 1.5% of the cases, respectively.[7]
Up to 4% of patients with typhoid fever go on to become chronic carriers. These patients remain asymptomatic after their acute treatment, but they may excrete Salmonella for up to 1 year in their stool, or less frequently their urine.[8] It is more commonly seen in women and those with biliary abnormalities, including cholelithiasis. Blood group antigens may also be linked to susceptibility to S. typhi chronic carriage.[9]
Pathophysiology
The pathogenesis of typhoid fever depends upon a number of factors, including infectious species, virulence, host's immunity, and infectious dose. The larger the infectious dose, the shorter the incubation period, and the higher the attack rate. Typhoid fever is more severe in debilitated and immunocompromised patients such as those with HIV (mainly paratyphi), those on glucocorticoid therapy, and those with altered phagocyte function (i.e., patients with malaria and sickle cell anemia). Salmonella is an acid-sensitive bacteria except for a few resistant strains, so typically it is destroyed in the stomach by gastric acid unless a large dose is ingested.[10] In patients with achlorhydria, intake of antacids and antihistamines, colonization of Salmonella occurs even with smaller doses. Food and beverages also act as buffers against gastric acid that facilitates bacteria reaching the small gut.[5]
The virulence of Salmonella is determined by typhoid toxin, Vi antigen (polysaccharide capsule), liposaccharide O antigen, and flagellar H antigen. Strains positive for Vi antigen have an attack rate twice that of Vi negative strains, even for the same dose of micro-organisms. One of the main differences between Salmonella typhi and non-typhoidal salmonella (NTS) is the presence of Vi antigen in Salmonella typhi but absent in NTS. The main role of the Vi antigen is to act as an antiphagocytic agent preventing the action of macrophages, thus shielding the O antigen from antibodies that confer the serum resistance. The flagellar H antigen provides bacterial mobility and adherence upon the gut wall mucosa. Invasion of the gut wall is assisted by flagella, and the type III secretion system is capable of transferring bacterial protein into enterocytes and M cells (specialized epithelial cells that serve as antigen-presenting cells in gut mucosa or lymphoid tissue) or by direct penetration of mucosa. Bacteria attached to M cells are absorbed by pinched off cytoplasm containing bacteria and extruded into the luminal space. In this process, M cells are damaged, and the basal lamina is exposed. It provides easy access to pathogens for the invasion, which worsens the condition.[11] The cystic fibrosis transmembrane conductance regulator (CFTR) is said to be important in the uptake of Salmonella; so, patients with abnormal CFTR protein are resistant to typhoid.[12] The transferred proteins activate the host cell Rho GTPases, which trigger the actin rearrangement so that bacterial protein uptake takes place in the phagosomes where the bacteria can grow. This special characteristic of the bacteria helps them to remain viable in a pool of host immunity. Salmonella also produces a molecule that stimulates the epithelial release of chemoattractant eicosanoid, which sequesters neutrophils into the lumen and potentiates mucosal damage.
Bacteria induce proliferation of Payer patches via recruitment of lymphocytes and mononuclear cells and induce necrosis and eventually, ulceration that complicates the symptoms. Pathogens reach the reticuloendothelial system via both lymphatic system and bloodstream, including other multiple organs, most commonly gallbladder in almost all cases. The early bacteremic phase (24 hours to 72 hours) is asymptomatic and transient as these bacteria are phagocytosed by macrophages and monocytes in the reticuloendothelial system called primary bacteremia. The capacity of pathogens to grow in these immune cells makes them characteristic, and intracellular multiplication of bacteria in the reticuloendothelial system enforces them to re-enter the bloodstream causing continuous bacteremia for several days and weeks known as secondary bacteremia. Secondary bacteremia is the phase in which disease symptoms manifest.[5] Like in other gram-negative bacteria, an endotoxin has an important role in the pathogenesis. The lipopolysaccharide induces the shock-like reaction, and endotoxemia leads to vascular hyperactivity and catecholamine release, which causes focal necrosis and hemorrhage.[13]
History and Physical
Clinical presentations of both Salmonella typhoid and Salmonella paratyphoid are similar, though arthralgia is more common with typhoid. Obtaining a history of permanent residence, travel history (travel to endemic and outbreak areas), immunization, socioeconomic status, lifestyle, onset and duration of illness, drug history (chemoprophylaxis of malaria, dose, and interval of the drug) are important to pave the way for diagnosis. Exposure history and related activities like impure drinking water, animal contact, insect bites, accommodations, undercooked food aid in excluding other infectious diseases. Typhoid is an infectious disease that presents with nonspecific symptoms. Patients complain of enterocolitis after 12 hours to 48 hours of inoculation. Often, they initially present with nausea, vomiting that progresses to diffuse abdominal pain, bloating, anorexia, and diarrhea (around 66%), which can vary from mild to severe diarrhea with or without blood, followed by a short asymptomatic phase that gives way to bacteremia and fever (about 96%) with flu-like symptoms.[14] Enterocolitis is more prominent in Salmonella typhi. Symptoms of enterocolitis generally last a few days and are self-limited without the need for medical intervention except in the old and very young. Immunocompromised patients with HIV, particularly those with low CD4 counts, more commonly present with severe diarrhea and tend to have more serious metastatic infections.[15] Classic typhoid fever starts about one week after the ingestion of the organism. Fever follows a “step-ladder” pattern (i.e., fever rises one day, falls the subsequent morning, and continues to form peaks and troughs with insidious onset). Abdominal distress is frequently seen in typhoid fever. Due to the hypertrophy of Payer patches, constipation may predominate over diarrhea in some cases.
Physical examination findings can be nonspecific. In the first week, a documented fever may be accompanied by a decreased heart rate. In the second week, findings are more common, including abdominal distention. When typhoid is complicated by ileal perforation, tenderness, rigidity, and guarding of the abdomen may be present. Visible rose spots (rose-colored macules on the abdomen) are associated with typhoid fever but occur only rarely. The patient looks pale, mildly distressed, and dehydrated with sunken eyes, dry skin, and lethargy. Some patients have jaundice with yellowish skin and sclera, pale stool, and dark urine when the patient has associated gallstones and other biliary pathology.[16] Enlarged spleen on palpation may also be present.
If the diagnosis is delayed until the third week, the patient is more toxic, anorexic, and with notable weight loss. Chances of bowel perforation increase with time, which worsens abdominal distension and peritonitis. The patient becomes tachypneic with crackles over the lung base on auscultation. Signs of metastatic complications appear. Dry cough due to pneumonia can also be present as well as neck rigidity due to meningitis, or rarely, chest pain due to myocarditis and pericarditis. Patients of endemic areas like India and Africa have more frequent neurologic manifestations like delirium, psychosis, insomnia, confusion, apathy, and in extremely rare cases, parkinsonism. Other unusual presentations are severe epigastric pain radiating to the back due to pancreatitis, bone pain because of osteomyelitis, and abscesses, which can occur anywhere in the body.
Evaluation
The approach to typhoid patients should be clinical. Patients residing in areas with poor sanitation or impure drinking water or history of travel from endemic areas presenting with febrile illness for more than three days along with gastrointestinal manifestations (pain, constipation, or diarrhea) are highly suspicious. Diagnosis in the first week is difficult, but a variety of laboratory studies assist in making the diagnosis.[17]
Blood culture: Blood culture remains the primary mechanism of confirmation of a typhoid fever diagnosis. It is widely available and the most commonly performed test, as it is not expensive or technically difficult. The efficacy of the blood culture is increased when high volume samples are taken. Blood cultures done during secondary bacteremia (i.e., clinical manifestations) are more reliable though 30% to 50% of cultures may be falsely negative depending on the technique and time series.[18]
Stool culture: Stool culture is less effective in the bacteremic phase of the disease. Stool culture is diagnostic in the second and third weeks. It was estimated to yield a positive result in only 37% of patients with antibiotic therapy.[19] The sensitivity of stool culture depends upon the amount of stool sample taken and the duration of illness. Chronic carriers intermittently pass pathogens in the fecal matter for a long time so, several samples should be taken. Additional metabolite biomarkers are under investigation.[20]
Bone marrow: Bone marrow culture is the gold standard for typhoid diagnosis.[21] The aspirated bone marrow sample is cultured in specific agar media. It is more sensitive than blood cultures due to the larger number of micro-organisms present in the bone marrow. Bone marrow culture is highly sensitive (around 90%) and even remains positive in more than 50% of cases despite several days of antibiotic therapy.[19] However, the test is highly invasive and expensive, so it is not routinely used for the diagnosis and treatment of typhoid.
Widal test: The Widal test is a serological test for enteric fever, which detects antibodies against O (surface) and H (flagellar) antigens. An antibody titer of greater than 1:160 and greater than 1:80 for anti-H antigen and anti-O antigen respectively are considered as cut off levels to predict recent infection of typhoid fever in an endemic area.[21] However, these cutoffs are dependent on the geographic area. When the convalescent titer is four times greater than the acute titer, the study is considered positive. Endemic areas will require higher titers to make the diagnosis and are still limited in that they may represent prior infection. The Widal test is not reliable due to its common false-negative and false-positive results, poor agreement with blood culture, and poor performance.
Skin snip test: Punch biopsies from characteristic rose spots may culture positive in up to 63% of the positive cases with prior therapeutic antibiotic treatment.[19]
Polymerase chain reaction (PCR) Assay: Polymerase Chain Reaction (PCR) can provide DNA-based gene identification of several serotypes such as the H antigen gene and O antigen gene.[22] However, sensitivities can be low due to low concentrations of bacteria during bacteremia. This testing is also cost-prohibitive in many low resource settings.
Enzyme-Linked Immunosorbent Assay (ELISA): ELISA identifies antibodies to the capsular polysaccharide Vi antigens that may be helpful in identifying carriers but is rarely useful in acute illness.[23]
Miscellaneous: Urine cultures and duodenal content culture via string capsule are not regularly performed, but may identify Salmonella typhi. Leukopenia and neutropenia are detectable in 15% to 25% of cases though leukocytosis can be seen as well, particularly in children. Liver function testing may show a pattern of viral hepatitis, and though nonspecific C-reactive protein may be elevated. When obtained, cerebrospinal fluid studies may reveal mild pleocytosis (less than 35cells), though most are unremarkable.[24] Electrocardiograms, ultrasound, liver enzymes and function tests, urine analysis, x-ray to evaluate air under the diaphragm are some additional evaluations that may be appropriate to diagnose other complications of the illness.
Treatment / Management
Antibiotic therapy is the mainstay of treatment. The treatment has been complicated by multidrug resistance strains developed in many endemic areas, especially in India and south-east Asia. The modality of treatment depends upon the severity of the disease, duration, dissemination, and complications.
Antibiotic therapy: Prompt administration of the relevant antibiotic therapy protects from severe complications of typhoid fever. Initial drug therapy of choice depends upon the susceptibility of the strains. In most areas, fluoroquinolones are the most effective drug of choice. In severe conditions that necessitate prompt treatment, fluoroquinolones can be administered empirically on clinical suspicion before the result of the diagnostic culture test. Fluoroquinolones cure about 98% of cases with relapse and fecal carriage rates of less than 2%. Ciprofloxacin (500 mg orally twice daily for 5-7 days) is the most effective fluoroquinolone. Amoxicillin (750mg orally 4 times daily for about 2 weeks), trimethoprim-sulfamethoxazole (160 mg twice daily for 2 weeks), and outside of the United States, chloramphenicol (500mg 4 times daily for 2-3 weeks) are all alternative treatments for adults in fully susceptible cases, but they are increasingly met with resistance. Uncomplicated cases can be managed at home with oral antibiotics and antipyretics. Patients with significant complications, including vomiting, diarrhea, and abdominal distension, should be hospitalized. Additional supportive therapy and parenteral antibiotics such as third-generation cephalosporins (guided by culture sensitivities) should be continued until 5 days after recovery. Multidrug-resistant (MDR) and extremely drug-resistant (XDR) strains have developed in endemic areas. The intracellular nature of bacteria safeguards against the extracellular antibiotics.[25] In MDR cases, third-generation cephalosporins (ceftriaxone, cefotaxime, and oral cefixime 2g once daily for 2 weeks) and azithromycin are the optimal treatment with ciprofloxacin as an alternative treatment. The failure rate of these therapies is nearly 5% to 10%, with relapse rate rates of 3% to 6%. These agents clear fever within a week with a fecal carriage rate of less than 3%. The addition of azithromycin and cefixime lowers the rate of failure and reduces the duration of hospitalization.[26]
Vaccination prophylaxis: Typhoid burden has been reduced since the invention of Salmonella typhi vaccination. The vaccine is recommended for those traveling to areas with risk of exposure. In the United States, there are two types of licensed, unconjugated vaccines.[27] The intramuscular Vi capsular polysaccharide vaccine is appropriate for those greater than two years old. It should be given 2 weeks or more before travel, and a booster should be provided every two years. A live attenuated oral vaccine (Ty21a strain of serotype Typhi) enhances immunity by stimulating the production of endogenous antibodies. It is indicated for those over 6 years old traveling to endemic areas or coming into close contact with chronic carriers or infected patients. It is done with a regimen of 4 capsules taken every other day with strict guidelines regarding the temperature of liquids used to ingest the capsule and ingestion on an empty stomach. It should be completed at least 1 week before exposure, and a booster is indicated every 5 years. As it is a live vaccine, the oral vaccine is not appropriate for pregnant patients or those with immunocompromised status. Though not licensed for this indication, the oral Ty21a vaccine may offer some protection against Salmonella paratyphi B. Both vaccines have similar efficacy at 50% to 80%, and travelers must practice avoidance measures in addition to the vaccine.
The World Health Organization Strategic Advisory Group of Experts on Immunization first recommended the use of typhoid conjugate vaccines (TCVs) in typhoid-endemic countries in 2017.[28] Intramuscular, single-dose TCVs for those 6 months and older are now registered in Nepal, India, Nigeria, and Cambodia and under further investigation for additional use in endemic areas and at times of outbreak.[29] When used in an outbreak of extensively drug-resistant typhoid in Pakistan in 2018, the TCV was found to be safe among children between 10 years and 6 months of age.[30] TCVs are also preferred due to the potential for a longer duration of immunity, safety in younger children, and the improved immunogenicity profile when compared to the Vi polysaccharide unconjugated vaccine.[31]
Miscellaneous treatment: Symptomatic and supportive care is essential. Maintaining adequate hydration during diarrhea, as well as appropriate ventilation and oxygenation for pulmonary complications, should be provided along with analgesics and antipyretics as supportive care for metastatic complications. Corticosteroids have been suggested for severe cases with encephalitis.[32]
Surgery: When gallstones accompany a carrier state, cholecystectomy can be curative.[33] Surgical repair is indicated for complications, including peritonitis and ileal perforation.[34]
Prevention through sanitation: Epidemiological data reveals that typhoid is more prevalent in low and middle-income countries, in areas with poor drinking water, and lack of sanitation. Safe drinking water, sanitation, and avoidance of overcrowding contribute remarkably to the reduction in the number of cases.
Differential Diagnosis
Typhoid fever has non-specific manifestations. It may resemble multiple infectious diseases with similar clinical presentations. So a wide differential diagnosis must be considered. Diseases with symptoms including diarrhea, dysentery, abdominal distension, fever, splenomegaly, and shock, should trigger consideration in the correct clinical context.
Dengue fever: Dengue is a hemorrhagic fever with non-specific symptoms like fever, headache, myalgia, shock, which may be confused with typhoid fever. It is known as “breakbone fever” for its severe arthralgias.[35]
Malaria: Malaria has non-specific clinical features such as fever, headache, myalgia, diarrhea, nausea, vomiting, and anemia. The involvement of multiple organs may make it difficult to differentiate it from typhoid clinically, but unlike typhoid, jaundice is common in malaria. Laboratory testing should rule out malaria in cases of fever in or after travel to endemic areas.
Amebiasis: Amebiasis is caused by Entamoeba histolytica ingested in water or uncooked food. Etiological factors such as impure drinking water and lack of sanitation should increase suspicion for amebiasis, similar to typhoid fever. Abdominal manifestations of amebiasis such as dysentery and liver abscess are common and may be difficult to distinguish from typhoid fever on a clinical basis alone.
Leptospirosis: It is one of the most common zoonotic diseases. It presents with fever and jaundice as well as features like myalgias, headaches, and conjunctival suffusion. Organisms spread throughout the body after brief bacteremia. Less common symptoms include cough, diarrhea, meningitis, acute kidney injury, hemorrhages, and macular rash.[36]
Q fever: Q fever (Coxiella burnetii infection) is a worldwide disease that presents with non-specific symptoms of fever that may include headache, chills, maculopapular rash, pneumonia, and osteomyelitis. Those in direct contact with cattle, sheep, and goats, such as ranchers and veterinarians, may be at higher risk of contracting Q fever.[37]
Tularemia: It is primarily a zoonotic disease prevalent in the northern hemisphere caused by a highly infectious gram-negative bacillus, Francisella tularensis. Though clinical features like hepatosplenomegaly, diarrhea vomiting, and pneumonia may be seen in typhoid fever, it is distinguished by skin ulceration with regional lymphadenopathy that is characteristic of tularemia.[38]
Melioidosis: Melioidosis or “Whitmore disease” is caused by Burkholderia pseudomallei and is seen more commonly in northern Australia and Southeast Asia, where it is passed to humans and animals via contact with contaminated water and soil. Melioidosis is most likely to present in patients with chronic diseases such as diabetes, renal and liver disease, thalassemia, chronic lung disease, and cancer. Pneumonia is the most common feature though other common features include hepatosplenomegaly, diarrhea, and skin abscesses, and ulcerations.[39]
Giardiasis: Giardia is a small intestine infection by the parasite Giardia lamblia. It is characterized by diarrhea, malaise abdominal cramps, and weight loss, but fever is typically absent or not prominent. It occurs worldwide and is common in the tropics transmitted by the waterborne, foodborne, and fecal-oral routes.
Bacterial gastroenteritis: A variety of other bacteria cause gastroenteritis with common clinical manifestations. These include Staphylococcus, Bacillus cereus, Clostridium perfringens, Campylobacter, Escherichia coli, Clostridium difficile, Vibrio cholerae, and bacillary dysentery (shigellosis).
Rickettsial infection: Rickettsia fever is characterized by fever with a rash caused by rickettsiae, a gram-negative bacteria. A skin lesion is prominent long with multi-systems manifestations.[40]
Toxoplasmosis: Toxoplasma gondii is an intracellular parasite causing toxoplasmosis. Enlarged spleen, lymphadenopathy, fever, malaise, sore throat, headache are the usual signs which may be self-limited or may even go unnoticed in immunocompetent individuals.
Tuberculosis: Tuberculosis (TB) is a common disease in developing countries. Fever associated with night sweats and weight loss is the characteristic feature that often leads to the diagnosis. Osteomyelitis in typhoid mimics Pott disease of the spine in TB.[41]
Brucellosis: Is an enzootic infection, similar to salmonella, in that it is also an intracellular organism that invades the reticuloendothelial system. Brucellosis spreads to humans typically from eating raw and unpasteurized dairy products or through contact with infected animals. Brucellosis presents with undulating fevers, fatigue, and arthralgias.[42]
Prognosis
Typhoid fever produces a major burden of mortality and morbidity worldwide, yet the problem is most prominent in south Asia and African countries. The overall current mortality rate has been reduced to less than 1% due to advancements in treatment modalities and the manufacture of antibiotics as compared to 12.75% in the 1940s when treatment was mainly symptomatic and supportive.[43] Early diagnosis and treatment avoid complications. Now, mortality is low despite the high frequency of episodes with complications.[44] In untreated patients, approximately 10% will relapse, and 4% will become chronic carriers.
Complications
As the primary site of Salmonella invasion is the gastrointestinal (GI) tract, gut complications are not surprising. Gastrointestinal irritation results in diarrhea, and Payer patch hypertrophy causes obstruction of lumen and constipation. In severe cases, necrosis of Payer patches leads to ulceration and bleeding. Sequelae of ulceration are eventually perforation of the terminal ileum. Diarrhea is usually non-bloody and loose. However, large-volume watery stools, bloody stools, and symptoms of dysentery may occur. The temperature may drop falsely at a normal or subnormal level due to intestinal hemorrhage.
While seeding can occur in nearly every organ system, it is uncommon to see complications outside of the GI tract. Widespread dissemination of bacteria causes multiorgan failure due to septicemia.[45] Hepatitis and encephalopathy may occur in 5% to 7% of the patients. Intraabdominal infections lead to hepatic and splenic abscesses. Pneumonia is less common. Other pulmonary complications include lung abscesses, empyemas, and bronchopleural fistula formation, though the majority of cases occur in patients with lung cancer, glucocorticoid use, and other structural lung diseases.[46]
Typhoid encephalopathy can occur in up to 17% of patients with a mortality as high as 55%.[47] While headaches are reported commonly, other neurologic symptoms, including sleep irregularities, acute psychosis, myelitis, meningitis, muscle rigidity, and focal neurologic deficits. Myocarditis and nephritis are the consequences of toxic phenomena.[48] Bone and joint infections occur more often in children with sickle cell anemia, hemoglobinopathies, and preexisting bone disease and most commonly affect the long bones, especially the femur, tibia, and humerus.[49] Joint involvement in these patients leads to septic arthritis. Patients with HLA-B27 antigens have a higher likelihood of reactive arthritis. Previously damaged organs, such as infarcts and aortic aneurysms, are the most frequent sites of the metastatic abscess. Complications are increased by prolonged duration of disease prior to hospitalization, long duration of hospitalization, techniques of antibiotic therapy, and immune compromise in debilitated patients with chronic diseases such as cancer, TB, and HIV. Nearly 3% of inadequately treated patients become chronic carriers. Patients that are inadequately treated for typhoid continue to excrete bacteria and are considered chronic carriers. The bacteria in the chronic stage of typhoid colonizes the gallbladder, and if not treated, can be linked to gallbladder cancer.[50][51]
Deterrence and Patient Education
While vaccines are available, they are only 50% to 80% effective in preventing the disease. Travelers must continue to practice good hygiene and take care to avoid exposure. Additional typhoid Vi conjugate vaccines have been approved in India and may become available soon with better efficacy. Typhoid fever is a global public health problem. Community health education about the mode of transmission, association with living standards, sanitation, prevention, signs and symptoms, the importance of early treatment, will not only reduce the prevalence of disease but also lower the healthcare workload. Counseling of patients about treatment modality and side effects is an important part of patient education.
Enhancing Healthcare Team Outcomes
Despite public health efforts, typhoid fever is still a significant cause of morbidity and mortality worldwide. Intersectoral coordination by other non-medical organizations and authorities in sanitation management, public healthcare awareness, and nutritional programs boost both the control and prevention of disease. Travelers to endemic areas should be advised to seek vaccination and to practice good food hygiene. A concerning trend is the emergence of extremely drug-resistant Salmonella typhi. Clinicians must encourage early care to establish the diagnosis and the correct antibiotic treatment that focuses on the appropriate choice of drug with adequate dosing and treatment duration to ensure patients face minimal complications. In times of outbreaks in endemic areas, coordinated public health campaigns swiftly administering vaccine and addressing sanitation concerns can decrease the burden of typhoid fever illness.[29] [Level 3]