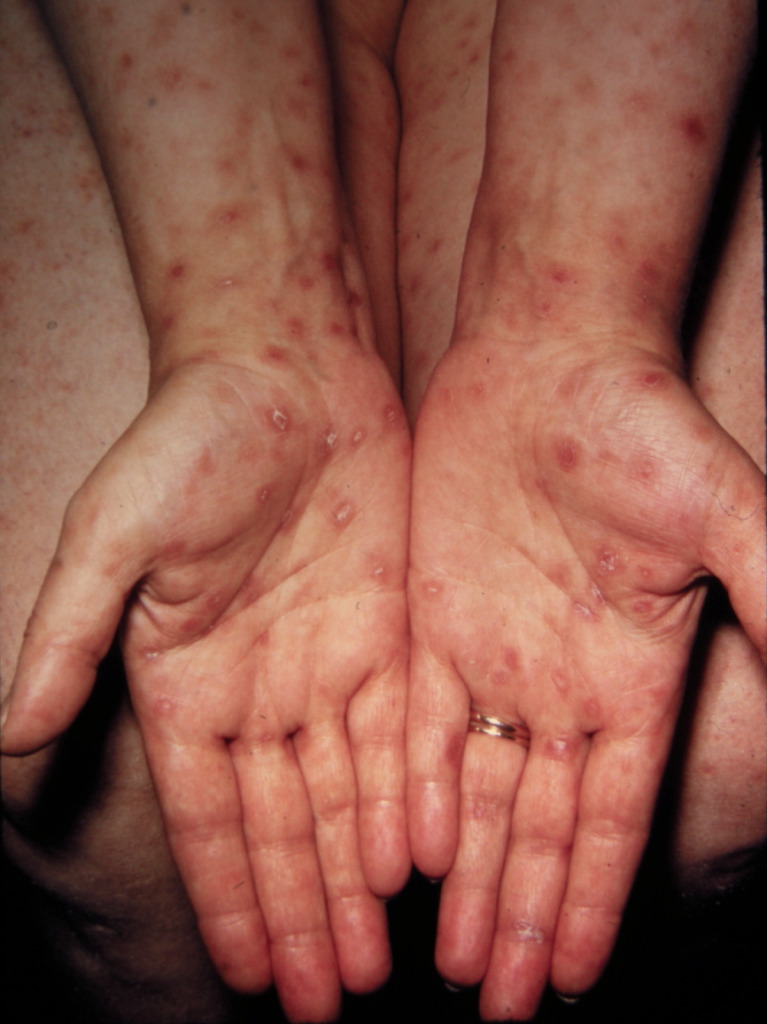
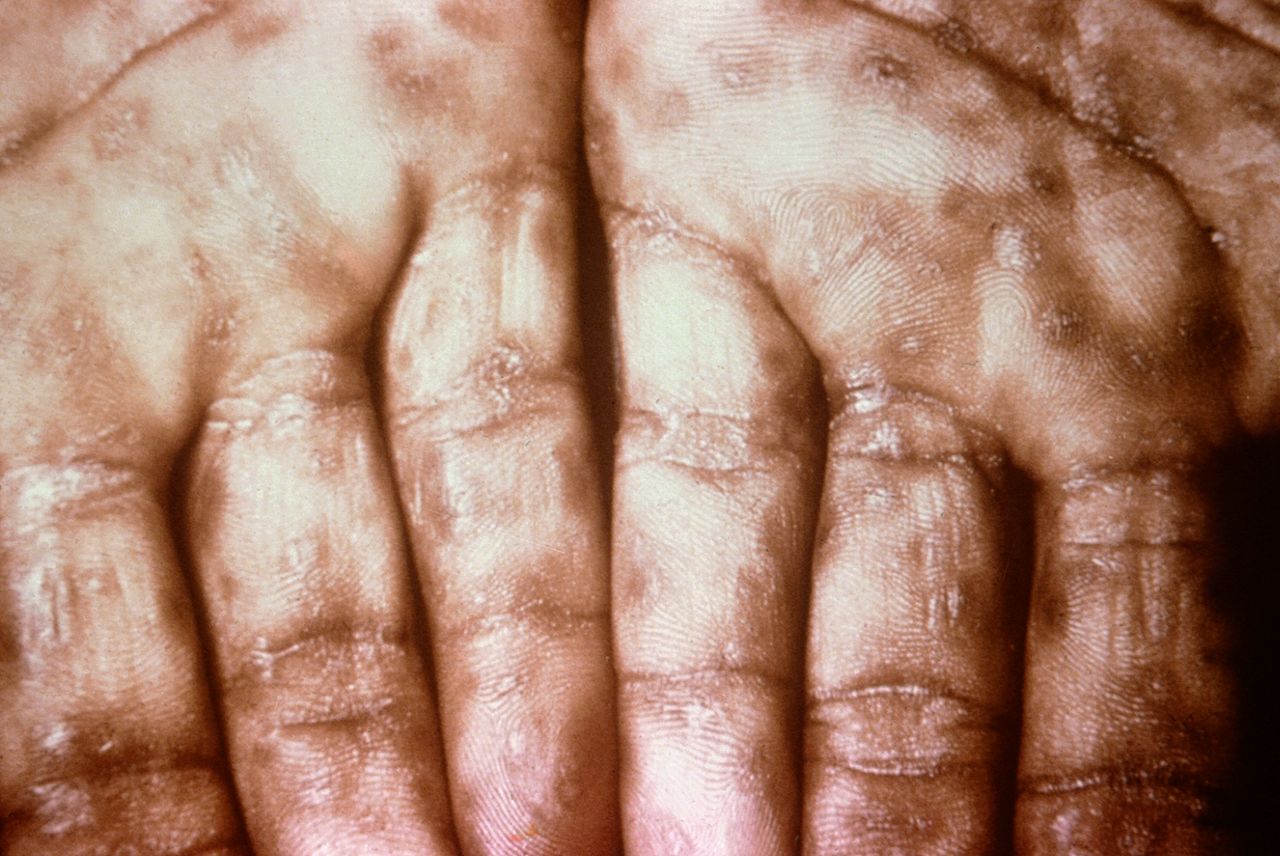
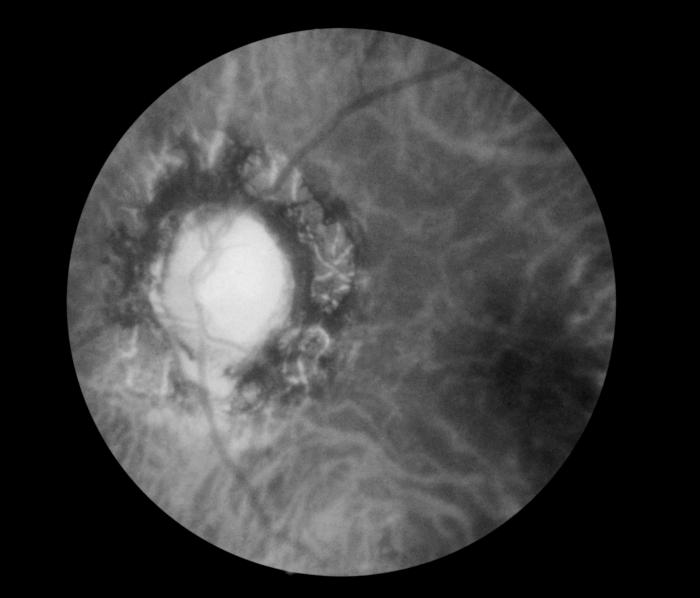
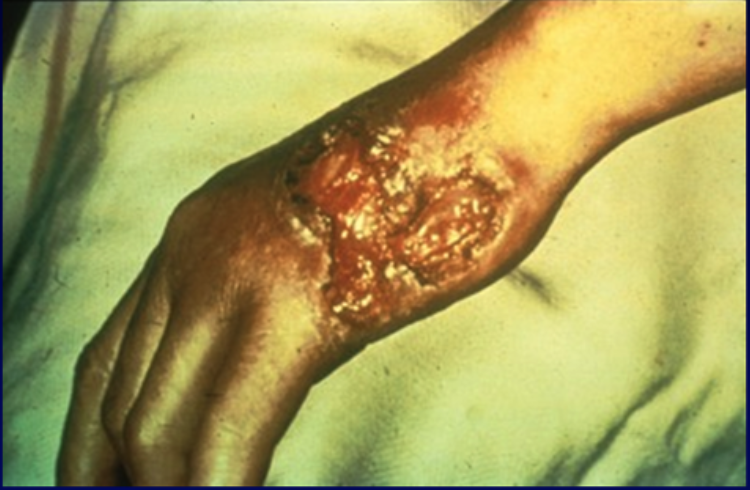
[1]
Anteric I, Basic Z, Vilovic K, Kolic K, Andjelinovic S. Which theory for the origin of syphilis is true? The journal of sexual medicine. 2014 Dec:11(12):3112-8. doi: 10.1111/jsm.12674. Epub 2014 Sep 4
[PubMed PMID: 25187322]
[2]
Lopez P, Kwentoh I, Valdez Imbert M. A Peculiar Presentation of Syphilis as a Mysterious Rash: A Dermatological Dilemma. Cureus. 2023 Sep:15(9):e45328. doi: 10.7759/cureus.45328. Epub 2023 Sep 15
[PubMed PMID: 37720122]
[3]
Tampa M, Sarbu I, Matei C, Benea V, Georgescu SR. Brief history of syphilis. Journal of medicine and life. 2014 Mar 15:7(1):4-10
[PubMed PMID: 24653750]
[4]
Beck A. The role of the spirochaete in the Wassermann reaction. The Journal of hygiene. 1939 May:39(3):298-310
[PubMed PMID: 20475495]
[5]
. THE WASSERMAN TEST FOR SYPHILIS IN PRACTICE. California state journal of medicine. 1910 Aug:8(8):252-3
[PubMed PMID: 18734999]
[6]
Gupta M, Verma GK, Sharma R, Sankhyan M, Rattan R, Negi AK. The Changing Trend of Syphilis: Is It a Sign of Impending Epidemic? Indian journal of dermatology. 2023 Jan-Feb:68(1):15-24. doi: 10.4103/ijd.ijd_788_22. Epub
[PubMed PMID: 37151273]
[7]
Kamat S, Vaghasia A, Dharmender J, Kansara KG, Shah BJ. Syphilis: Is it Back with a Bang? Indian dermatology online journal. 2024 Jan-Feb:15(1):73-77. doi: 10.4103/idoj.idoj_187_23. Epub 2023 Dec 22
[PubMed PMID: 38283002]
[8]
Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. The Lancet. Infectious diseases. 2004 Jul:4(7):456-66
[PubMed PMID: 15219556]
[9]
Ren M, Dashwood T, Walmsley S. The Intersection of HIV and Syphilis: Update on the Key Considerations in Testing and Management. Current HIV/AIDS reports. 2021 Aug:18(4):280-288. doi: 10.1007/s11904-021-00564-z. Epub 2021 Jun 6
[PubMed PMID: 34091858]
[10]
Refugio ON, Klausner JD. Syphilis incidence in men who have sex with men with human immunodeficiency virus comorbidity and the importance of integrating sexually transmitted infection prevention into HIV care. Expert review of anti-infective therapy. 2018 Apr:16(4):321-331. doi: 10.1080/14787210.2018.1446828. Epub 2018 Mar 5
[PubMed PMID: 29489420]
[12]
Park IU, Tran A, Pereira L, Fakile Y. Sensitivity and Specificity of Treponemal-specific Tests for the Diagnosis of Syphilis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2020 Jun 24:71(Suppl 1):S13-S20. doi: 10.1093/cid/ciaa349. Epub
[PubMed PMID: 32578866]
[13]
Peeling RW, Hook EW 3rd. The pathogenesis of syphilis: the Great Mimicker, revisited. The Journal of pathology. 2006 Jan:208(2):224-32
[PubMed PMID: 16362988]
[14]
Chen T, Wan B, Wang M, Lin S, Wu Y, Huang J. Evaluating the global, regional, and national impact of syphilis: results from the global burden of disease study 2019. Scientific reports. 2023 Jul 14:13(1):11386. doi: 10.1038/s41598-023-38294-4. Epub 2023 Jul 14
[PubMed PMID: 37452074]
[15]
Rowley J, Vander Hoorn S, Korenromp E, Low N, Unemo M, Abu-Raddad LJ, Chico RM, Smolak A, Newman L, Gottlieb S, Thwin SS, Broutet N, Taylor MM. Chlamydia, gonorrhoea, trichomoniasis and syphilis: global prevalence and incidence estimates, 2016. Bulletin of the World Health Organization. 2019 Aug 1:97(8):548-562P. doi: 10.2471/BLT.18.228486. Epub 2019 Jun 6
[PubMed PMID: 31384073]
[16]
Byard RW, Syphilis-Cardiovascular Manifestations of the Great Imitator. Journal of forensic sciences. 2018 Jul;
[PubMed PMID: 29193072]
[17]
Hook EW 3rd. Syphilis. Lancet (London, England). 2017 Apr 15:389(10078):1550-1557. doi: 10.1016/S0140-6736(16)32411-4. Epub 2016 Dec 18
[PubMed PMID: 27993382]
[18]
Storey A, Seghers F, Pyne-Mercier L, Peeling RW, Owiredu MN, Taylor MM. Syphilis diagnosis and treatment during antenatal care: the potential catalytic impact of the dual HIV and syphilis rapid diagnostic test. The Lancet. Global health. 2019 Aug:7(8):e1006-e1008. doi: 10.1016/S2214-109X(19)30248-7. Epub
[PubMed PMID: 31303285]
[19]
Whiting C, Schwartzman G, Khachemoune A. Syphilis in Dermatology: Recognition and Management. American journal of clinical dermatology. 2023 Mar:24(2):287-297. doi: 10.1007/s40257-022-00755-3. Epub 2023 Jan 23
[PubMed PMID: 36689103]
[20]
Kimball A, Torrone E, Miele K, Bachmann L, Thorpe P, Weinstock H, Bowen V. Missed Opportunities for Prevention of Congenital Syphilis - United States, 2018. MMWR. Morbidity and mortality weekly report. 2020 Jun 5:69(22):661-665. doi: 10.15585/mmwr.mm6922a1. Epub 2020 Jun 5
[PubMed PMID: 32497029]
[21]
Kidd SE, Grey JA, Torrone EA, Weinstock HS. Increased Methamphetamine, Injection Drug, and Heroin Use Among Women and Heterosexual Men with Primary and Secondary Syphilis - United States, 2013-2017. MMWR. Morbidity and mortality weekly report. 2019 Feb 15:68(6):144-148. doi: 10.15585/mmwr.mm6806a4. Epub 2019 Feb 15
[PubMed PMID: 30763294]
[22]
Spiteri G, Unemo M, Mårdh O, Amato-Gauci AJ. The resurgence of syphilis in high-income countries in the 2000s: a focus on Europe. Epidemiology and infection. 2019 Jan:147():e143. doi: 10.1017/S0950268819000281. Epub
[PubMed PMID: 30869043]
[23]
Korenromp EL, Rowley J, Alonso M, Mello MB, Wijesooriya NS, Mahiané SG, Ishikawa N, Le LV, Newman-Owiredu M, Nagelkerke N, Newman L, Kamb M, Broutet N, Taylor MM. Global burden of maternal and congenital syphilis and associated adverse birth outcomes-Estimates for 2016 and progress since 2012. PloS one. 2019:14(2):e0211720. doi: 10.1371/journal.pone.0211720. Epub 2019 Feb 27
[PubMed PMID: 30811406]
[24]
Newman L, Rowley J, Vander Hoorn S, Wijesooriya NS, Unemo M, Low N, Stevens G, Gottlieb S, Kiarie J, Temmerman M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PloS one. 2015:10(12):e0143304. doi: 10.1371/journal.pone.0143304. Epub 2015 Dec 8
[PubMed PMID: 26646541]
Level 1 (high-level) evidence
[25]
Gilmour LS, Walls T. Congenital Syphilis: a Review of Global Epidemiology. Clinical microbiology reviews. 2023 Jun 21:36(2):e0012622. doi: 10.1128/cmr.00126-22. Epub 2023 Mar 15
[PubMed PMID: 36920205]
[26]
Sankaran D, Partridge E, Lakshminrusimha S. Congenital Syphilis-An Illustrative Review. Children (Basel, Switzerland). 2023 Jul 29:10(8):. doi: 10.3390/children10081310. Epub 2023 Jul 29
[PubMed PMID: 37628309]
[27]
Gregory ECW, Ely DM. Trends and Characteristics in Maternal Syphilis Rates During Pregnancy: United States, 2016-2022. NCHS data brief. 2024 Feb:(496):1-8
[PubMed PMID: 38358322]
[28]
Fang J, Partridge E, Bautista GM, Sankaran D. Congenital Syphilis Epidemiology, Prevention, and Management in the United States: A 2022 Update. Cureus. 2022 Dec:14(12):e33009. doi: 10.7759/cureus.33009. Epub 2022 Dec 27
[PubMed PMID: 36712768]
[29]
National Academies of Sciences, Engineering, and Medicine;Health and Medicine Division;Board on Population Health and Public Health Practice;Committee on Prevention and Control of Sexually Transmitted Infections in the United States, Crowley JS, Geller AB, Vermund SH. Sexually Transmitted Infections: Adopting a Sexual Health Paradigm. 2021 Mar 24:():
[PubMed PMID: 34432397]
[30]
Park E, Yip J, Harville E, Nelson M, Giarratano G, Buekens P, Wagman J. Gaps in the congenital syphilis prevention cascade: qualitative findings from Kern County, California. BMC infectious diseases. 2022 Feb 5:22(1):129. doi: 10.1186/s12879-022-07100-3. Epub 2022 Feb 5
[PubMed PMID: 35123425]
Level 2 (mid-level) evidence
[31]
Paixao ES, Ferreira AJF, Pescarini JM, Wong KLM, Goes E, Fiaccone R, Lopes de Oliveira G, Reboucas P, Cardoso AM, Smeeth L, Barreto ML, Rodrigues LC, Ichihara MY. Maternal and congenital syphilis attributable to ethnoracial inequalities: a national record-linkage longitudinal study of 15 million births in Brazil. The Lancet. Global health. 2023 Nov:11(11):e1734-e1742. doi: 10.1016/S2214-109X(23)00405-9. Epub
[PubMed PMID: 37858584]
[32]
Crenner C. The Tuskegee Syphilis Study and the scientific concept of racial nervous resistance. Journal of the history of medicine and allied sciences. 2012 Apr:67(2):244-80. doi: 10.1093/jhmas/jrr003. Epub 2011 Feb 12
[PubMed PMID: 21317423]
[33]
McCallum JM, Arekere DM, Green BL, Katz RV, Rivers BM. Awareness and knowledge of the U.S. Public Health Service syphilis study at Tuskegee: implications for biomedical research. Journal of health care for the poor and underserved. 2006 Nov:17(4):716-33
[PubMed PMID: 17242526]
[34]
Gurnham D. Principles, progress and harm in the Guatemala Syphilis Study. Sexually transmitted infections. 2013 Jun:89(4):303. doi: 10.1136/sextrans-2013-051113. Epub
[PubMed PMID: 23687133]
[35]
Zenilman JM. Ethics gone awry: the US Public Health Service studies in Guatemala; 1946-1948. Sexually transmitted infections. 2013 Jun:89(4):295-300. doi: 10.1136/sextrans-2012-050741. Epub
[PubMed PMID: 23687131]
[36]
Reverby SM. Will the STI studies in Guatemala be remembered, and for what? Sexually transmitted infections. 2013 Jun:89(4):301-2. doi: 10.1136/sextrans-2013-051115. Epub
[PubMed PMID: 23687132]
[37]
Jongen VW, Van Der Loeff MFS, Van Den Elshout M, Wijstma E, Coyer L, Davidovich U, De Vries HJC, Prins M, Hoornenborg E, Boyd A. Bacterial sexually transmitted infections are concentrated in subpopulations of men who have sex with men using HIV pre-exposure prophylaxis. AIDS (London, England). 2023 Nov 1:37(13):2059-2068. doi: 10.1097/QAD.0000000000003676. Epub 2023 Jul 27
[PubMed PMID: 37503635]
[38]
Martín-Sánchez M, Case R, Fairley C, Hocking JS, Bradshaw C, Ong J, Chen MY, Chow EPF. Trends and differences in sexual practices and sexually transmitted infections in men who have sex with men only (MSMO) and men who have sex with men and women (MSMW): a repeated cross-sectional study in Melbourne, Australia. BMJ open. 2020 Nov 24:10(11):e037608. doi: 10.1136/bmjopen-2020-037608. Epub 2020 Nov 24
[PubMed PMID: 33234617]
Level 2 (mid-level) evidence
[39]
Ghanem KG, Ram S, Rice PA. The Modern Epidemic of Syphilis. The New England journal of medicine. 2020 Feb 27:382(9):845-854. doi: 10.1056/NEJMra1901593. Epub
[PubMed PMID: 32101666]
[40]
Solomon MM, Mayer KH. Evolution of the syphilis epidemic among men who have sex with men. Sexual health. 2015 Apr:12(2):96-102. doi: 10.1071/SH14173. Epub
[PubMed PMID: 25514173]
[41]
Mashingaidze R, Moodie Z, Allen M, Bekker LG, Grove D, Grunenberg N, Huang Y, Janes HE, Lazarus EM, Malahleha M, Nchabeleng M, Laher F. Sexually transmitted infections amongst men who have sex with men (MSM) in South Africa. PLOS global public health. 2023:3(4):e0001782. doi: 10.1371/journal.pgph.0001782. Epub 2023 Apr 5
[PubMed PMID: 37018240]
[42]
Hung P, Osias E, Konda KA, Calvo GM, Reyes-Díaz EM, Vargas SK, Goldbeck C, Caceres CF, Klausner JD. High Lifetime Prevalence of Syphilis in Men Who Have Sex With Men and Transgender Women Versus Low Lifetime Prevalence in Female Sex Workers in Lima, Peru. Sexually transmitted diseases. 2020 Aug:47(8):549-555. doi: 10.1097/OLQ.0000000000001200. Epub
[PubMed PMID: 32541611]
[43]
Vergara-Ortega DN, Tapia-Maltos A, Herrera-Ortíz A, García-Cisneros S, Olamendi-Portugal M, Sánchez-Alemán MÁ. High Prevalence of Syphilis and Syphilis/HIV Coinfection among Men Who Have Sex with Men Who Attend Meeting Places in Mexico. Pathogens (Basel, Switzerland). 2023 Feb 21:12(3):. doi: 10.3390/pathogens12030356. Epub 2023 Feb 21
[PubMed PMID: 36986278]
[44]
Zhao T, Chen G, Sun C, Gong X, Li H, Fu G. The epidemic of HIV and syphilis and the correlation with substance abuse among men who have sex with men in China: A systematic review and meta-analysis. Frontiers in public health. 2023:11():1082637. doi: 10.3389/fpubh.2023.1082637. Epub 2023 Feb 17
[PubMed PMID: 36875380]
Level 1 (high-level) evidence
[45]
Peterman TA, Su J, Bernstein KT, Weinstock H. Syphilis in the United States: on the rise? Expert review of anti-infective therapy. 2015 Feb:13(2):161-8. doi: 10.1586/14787210.2015.990384. Epub 2014 Dec 9
[PubMed PMID: 25487961]
[46]
Tsuboi M, Evans J, Davies EP, Rowley J, Korenromp EL, Clayton T, Taylor MM, Mabey D, Chico RM. Prevalence of syphilis among men who have sex with men: a global systematic review and meta-analysis from 2000-20. The Lancet. Global health. 2021 Aug:9(8):e1110-e1118. doi: 10.1016/S2214-109X(21)00221-7. Epub 2021 Jul 8
[PubMed PMID: 34246332]
Level 1 (high-level) evidence
[47]
Gray RT, Hoare A, Prestage GP, Donovan B, Kaldor JM, Wilson DP. Frequent testing of highly sexually active gay men is required to control syphilis. Sexually transmitted diseases. 2010 May:37(5):298-305. doi: 10.1097/OLQ.0b013e3181ca3c0a. Epub
[PubMed PMID: 20393383]
[48]
Wong W, Chaw JK, Kent CK, Klausner JD. Risk factors for early syphilis among gay and bisexual men seen in an STD clinic: San Francisco, 2002-2003. Sexually transmitted diseases. 2005 Jul:32(7):458-63
[PubMed PMID: 15976605]
[49]
Wu Y, Zhu W, Sun C, Yue X, Zheng M, Fu G, Gong X. Prevalence of syphilis among people living with HIV and its implication for enhanced coinfection monitoring and management in China: A meta-analysis. Frontiers in public health. 2022:10():1002342. doi: 10.3389/fpubh.2022.1002342. Epub 2022 Oct 17
[PubMed PMID: 36324449]
Level 1 (high-level) evidence
[50]
Traeger MW, Cornelisse VJ, Asselin J, Price B, Roth NJ, Willcox J, Tee BK, Fairley CK, Chang CC, Armishaw J, Vujovic O, Penn M, Cundill P, Forgan-Smith G, Gall J, Pickett C, Lal L, Mak A, Spelman TD, Nguyen L, Murphy DA, Ryan KE, El-Hayek C, West M, Ruth S, Batrouney C, Lockwood JT, Hoy JF, Hellard ME, Stoové MA, Wright EJ, PrEPX Study Team. Association of HIV Preexposure Prophylaxis With Incidence of Sexually Transmitted Infections Among Individuals at High Risk of HIV Infection. JAMA. 2019 Apr 9:321(14):1380-1390. doi: 10.1001/jama.2019.2947. Epub
[PubMed PMID: 30964528]
[51]
Azarnoosh M, Johansen IS, Martin-Iguacel R. Incidence of Sexually Transmitted Infections After Initiating HIV Pre-Exposure Prophylaxis Among MSM in Southern Denmark. American journal of men's health. 2021 May-Jun:15(3):15579883211018917. doi: 10.1177/15579883211018917. Epub
[PubMed PMID: 34036826]
[52]
Pathela P, Braunstein SL, Schillinger JA, Shepard C, Sweeney M, Blank S. Men who have sex with men have a 140-fold higher risk for newly diagnosed HIV and syphilis compared with heterosexual men in New York City. Journal of acquired immune deficiency syndromes (1999). 2011 Dec 1:58(4):408-16. doi: 10.1097/QAI.0b013e318230e1ca. Epub
[PubMed PMID: 21857351]
[53]
Schmidt R, Carson PJ, Jansen RJ. Resurgence of Syphilis in the United States: An Assessment of Contributing Factors. Infectious diseases. 2019:12():1178633719883282. doi: 10.1177/1178633719883282. Epub 2019 Oct 16
[PubMed PMID: 31666795]
[54]
Ramchandani MS, Cannon CA, Marra CM. Syphilis: A Modern Resurgence. Infectious disease clinics of North America. 2023 Jun:37(2):195-222. doi: 10.1016/j.idc.2023.02.006. Epub 2023 Mar 31
[PubMed PMID: 37005164]
[55]
McKetin R, Lubman DI, Baker A, Dawe S, Ross J, Mattick RP, Degenhardt L. The relationship between methamphetamine use and heterosexual behaviour: evidence from a prospective longitudinal study. Addiction (Abingdon, England). 2018 Jul:113(7):1276-1285. doi: 10.1111/add.14181. Epub 2018 Mar 12
[PubMed PMID: 29397001]
[56]
Kenyon CR, Osbak K, Tsoumanis A. The Global Epidemiology of Syphilis in the Past Century - A Systematic Review Based on Antenatal Syphilis Prevalence. PLoS neglected tropical diseases. 2016 May:10(5):e0004711. doi: 10.1371/journal.pntd.0004711. Epub 2016 May 11
[PubMed PMID: 27167068]
Level 1 (high-level) evidence
[57]
Kenyon C, Lynen L, Florence E, Caluwaerts S, Vandenbruaene M, Apers L, Soentjens P, Van Esbroeck M, Bottieau E. Syphilis reinfections pose problems for syphilis diagnosis in Antwerp, Belgium - 1992 to 2012. Euro surveillance : bulletin Europeen sur les maladies transmissibles = European communicable disease bulletin. 2014 Nov 13:19(45):20958
[PubMed PMID: 25411690]
[58]
Kaur G, Kaur P. Syphilis testing in blood donors: an update. Blood transfusion = Trasfusione del sangue. 2015 Apr:13(2):197-204. doi: 10.2450/2014.0146-14. Epub 2014 Nov 21
[PubMed PMID: 25545876]
[59]
Gardella C, Marfin AA, Kahn RH, Swint E, Markowitz LE. Persons with early syphilis identified through blood or plasma donation screening in the United States. The Journal of infectious diseases. 2002 Feb 15:185(4):545-9
[PubMed PMID: 11865408]
[60]
Owusu-Ofori AK, Parry CM, Bates I. Transfusion-transmitted syphilis in teaching hospital, Ghana. Emerging infectious diseases. 2011 Nov:17(11):2080-2. doi: 10.3201/eid1711.110985. Epub
[PubMed PMID: 22099108]
[61]
Adegoke AO, Akanni O, Dirisu J. Risk of transfusion-transmitted syphilis in a tertiary hospital in Nigeria. North American journal of medical sciences. 2011 Feb:3(2):78-81. doi: 10.4297/najms.2011.378. Epub
[PubMed PMID: 22540070]
[62]
Peeling RW, Mabey D, Chen XS, Garcia PJ. Syphilis. Lancet (London, England). 2023 Jul 22:402(10398):336-346. doi: 10.1016/S0140-6736(22)02348-0. Epub
[PubMed PMID: 37481272]
[63]
Sadoghi B, Stary G, Wolf P. Syphilis. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2023 May:21(5):504-517. doi: 10.1111/ddg.14999. Epub
[PubMed PMID: 37183747]
[64]
Mullally D, Kotha S, Mandour MO, Berry P. Luetic (syphilitic) hepatitis: the great imitator persists in the 21st century. BMJ case reports. 2023 May 9:16(5):. doi: 10.1136/bcr-2022-254330. Epub 2023 May 9
[PubMed PMID: 37160377]
Level 3 (low-level) evidence
[65]
Ashton R, Robern M. Syphilis: The great imitator. Canadian family physician Medecin de famille canadien. 2023 Oct:69(10):697-698. doi: 10.46747/cfp.6910697. Epub
[PubMed PMID: 37833082]
[66]
Finney N, Soyfer E, Schwan R, Spiegelman LC. The Continued Rise of Syphilis: A Case Report to Aid in Identification of the Great Imitator. Journal of education & teaching in emergency medicine. 2023 Apr:8(2):V11-V15. doi: 10.21980/J8KM02. Epub 2023 Apr 30
[PubMed PMID: 37465660]
Level 3 (low-level) evidence
[67]
Kaur B, Khanna D. A Narrative Review of the Many Psychiatric Manifestations of Neurosyphilis: The Great Imitator. Cureus. 2023 Sep:15(9):e44866. doi: 10.7759/cureus.44866. Epub 2023 Sep 7
[PubMed PMID: 37814742]
Level 3 (low-level) evidence
[68]
Culbert AA, Israel AK, Ku J, Silver NL. The Increasing Problem of Syphilis Manifesting as Head and Neck Cancer: A Case Series. The Laryngoscope. 2024 Jan:134(1):236-239. doi: 10.1002/lary.30765. Epub 2023 May 29
[PubMed PMID: 37246718]
Level 2 (mid-level) evidence
[69]
Ciepłucha H, Bożejko M, Knysz B, Inglot M, Leszczyszyn J. The great imitator: potential problems in the diagnostics of clinical manifestations of syphilis. Polish archives of internal medicine. 2023 Mar 1:133(3):. pii: 16420. doi: 10.20452/pamw.16420. Epub 2023 Jan 24
[PubMed PMID: 36734955]
[72]
Edmondson DG, Hu B, Norris SJ. Long-Term In Vitro Culture of the Syphilis Spirochete Treponema pallidum subsp. pallidum. mBio. 2018 Jun 26:9(3):. doi: 10.1128/mBio.01153-18. Epub 2018 Jun 26
[PubMed PMID: 29946052]
[73]
Edmondson DG, Norris SJ. In Vitro Cultivation of the Syphilis Spirochete Treponema pallidum. Current protocols. 2021 Feb:1(2):e44. doi: 10.1002/cpz1.44. Epub
[PubMed PMID: 33599121]
[75]
Levy JA. Confirmation of the successful cultivation of Treponema pallidum in tissue culture. Microbiologica. 1984 Oct:7(4):367-70
[PubMed PMID: 6392830]
[76]
Radolf JD, Deka RK, Anand A, Šmajs D, Norgard MV, Yang XF. Treponema pallidum, the syphilis spirochete: making a living as a stealth pathogen. Nature reviews. Microbiology. 2016 Dec:14(12):744-759. doi: 10.1038/nrmicro.2016.141. Epub 2016 Oct 10
[PubMed PMID: 27721440]
[77]
Radolf JD, Desrosiers DC. Treponema pallidum, the stealth pathogen, changes, but how? Molecular microbiology. 2009 Jun:72(5):1081-6. doi: 10.1111/j.1365-2958.2009.06711.x. Epub 2009 May 4
[PubMed PMID: 19432802]
[78]
Salazar JC, Hazlett KR, Radolf JD. The immune response to infection with Treponema pallidum, the stealth pathogen. Microbes and infection. 2002 Sep:4(11):1133-40
[PubMed PMID: 12361913]
[79]
Pierce EF, Katz KA. Darkfield microscopy for point-of-care syphilis diagnosis. MLO: medical laboratory observer. 2011 Jan:43(1):30-1
[PubMed PMID: 21309351]
[80]
Flamm A, Parikh K, Xie Q, Kwon EJ, Elston DM. Histologic features of secondary syphilis: A multicenter retrospective review. Journal of the American Academy of Dermatology. 2015 Dec:73(6):1025-30. doi: 10.1016/j.jaad.2015.08.062. Epub 2015 Oct 20
[PubMed PMID: 26464219]
Level 2 (mid-level) evidence
[81]
Centurion-Lara A, Castro C, Shaffer JM, Van Voorhis WC, Marra CM, Lukehart SA. Detection of Treponema pallidum by a sensitive reverse transcriptase PCR. Journal of clinical microbiology. 1997 Jun:35(6):1348-52
[PubMed PMID: 9163442]
[82]
Centurion-Lara A, Castro C, van Voorhis WC, Lukehart SA. Two 16S-23S ribosomal DNA intergenic regions in different Treponema pallidum subspecies contain tRNA genes. FEMS microbiology letters. 1996 Oct 1:143(2-3):235-40
[PubMed PMID: 8837477]
Level 3 (low-level) evidence
[83]
Elmouden H, Louhab N, Kissani N. Medullary involvement in neurosyphilis: a report of 12 cases and a review of the literature. Spinal cord series and cases. 2019:5():38. doi: 10.1038/s41394-019-0185-9. Epub 2019 May 1
[PubMed PMID: 31632699]
Level 3 (low-level) evidence
[84]
Chapel TA. The signs and symptoms of secondary syphilis. Sexually transmitted diseases. 1980 Oct-Dec:7(4):161-4
[PubMed PMID: 7455863]
[85]
Pleimes M, Hartschuh W, Kutzner H, Enk AH, Hartmann M. Malignant syphilis with ocular involvement and organism-depleted lesions. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Jan 1:48(1):83-5. doi: 10.1086/594127. Epub
[PubMed PMID: 19035775]
[86]
Ehlers S, Sergent S, Ashurst J. Secondary Syphilis. Clinical practice and cases in emergency medicine. 2020 Nov:4(4):675-676. doi: 10.5811/cpcem.2020.7.48515. Epub
[PubMed PMID: 33217306]
Level 3 (low-level) evidence
[87]
Gjestland T. The Oslo study of untreated syphilis; an epidemiologic investigation of the natural course of the syphilitic infection based upon a re-study of the Boeck-Bruusgaard material. Acta dermato-venereologica. Supplementum. 1955:35(Suppl 34):3-368; Annex I-LVI
[PubMed PMID: 13301322]
[88]
Pourang A, Fung MA, Tartar D, Brassard A. Condyloma lata in secondary syphilis. JAAD case reports. 2021 Apr:10():18-21. doi: 10.1016/j.jdcr.2021.01.025. Epub 2021 Feb 9
[PubMed PMID: 33732839]
Level 3 (low-level) evidence
[89]
Magdaleno-Tapial J, Hernández-Bel P, Valenzuela-Oñate C, Miquel VA. Whitish Dots Provide the Key to Diagnosing Condyloma Lata: A Report of 5 Cases. Actas dermo-sifiliograficas. 2020 Dec:111(10):892-895. doi: 10.1016/j.ad.2019.02.020. Epub 2020 Jun 20
[PubMed PMID: 32574714]
Level 3 (low-level) evidence
[90]
Mindel A, Tovey SJ, Timmins DJ, Williams P. Primary and secondary syphilis, 20 years' experience. 2. Clinical features. Genitourinary medicine. 1989 Jan:65(1):1-3
[PubMed PMID: 2921046]
[91]
Minkin W, Landy SE, Cohen HJ. An unusual solitary lesion of secondary syphilis. Archives of dermatology. 1967 Feb:95(2):217
[PubMed PMID: 6019000]
[92]
Pavithran K. Solitary condyloma latum on the umbilicus. International journal of dermatology. 1992 Aug:31(8):597-8
[PubMed PMID: 1428457]
[93]
Liu Z, Wang L, Zhang G, Long H. Warty mucosal lesions: Oral condyloma lata of secondary syphilis. Indian journal of dermatology, venereology and leprology. 2017 Mar-Apr:83(2):277. doi: 10.4103/0378-6323.191129. Epub
[PubMed PMID: 27679405]
[94]
D'Amico R, Zalusky R. A case of lues maligna in a patient with acquired immunodeficiency syndrome (AIDS). Scandinavian journal of infectious diseases. 2005:37(9):697-700
[PubMed PMID: 16126575]
Level 3 (low-level) evidence
[95]
Petrozzi JW, Lockshin NA, Berger BJ. Malignant syphilis. Severe variant of secondary syphilis. Archives of dermatology. 1974 Mar:109(3):387-9
[PubMed PMID: 4592567]
[96]
Tucker JD, Shah S, Jarell AD, Tsai KY, Zembowicz A, Kroshinsky D. Lues maligna in early HIV infection case report and review of the literature. Sexually transmitted diseases. 2009 Aug:36(8):512-4. doi: 10.1097/OLQ.0b013e3181a2a946. Epub
[PubMed PMID: 19455078]
Level 3 (low-level) evidence
[97]
Wibisono O, Idrus I, Djawad K. Malignant Syphilis: A Systematic Review of the Case Reports Published in 2014-2018. Actas dermo-sifiliograficas. 2021 Mar 31:():. pii: S0001-7310(21)00135-6. doi: 10.1016/j.ad.2021.02.011. Epub 2021 Mar 31
[PubMed PMID: 33811833]
Level 1 (high-level) evidence
[98]
Correia C, Borges-Costa J, Soares-de-Almeida L, Filipe P. Lues Maligna in an Immunocompetent Patient. Sexually transmitted diseases. 2022 Dec 1:49(12):863-864. doi: 10.1097/OLQ.0000000000001664. Epub 2022 Jun 17
[PubMed PMID: 35709215]
[99]
Margulies S, Patel SP, Motaparthi K. Ulceronecrotic rash in an immunocompetent individual. JAAD case reports. 2022 Sep:27():29-31. doi: 10.1016/j.jdcr.2022.06.029. Epub 2022 Jul 5
[PubMed PMID: 35990229]
Level 3 (low-level) evidence
[100]
Demirbaş A, Şikar Aktürk A, Odyakmaz Demirsoy E, Kıran R, Bayramgürler D, Sayman N, Açıkbaş E, Vural Ç. Lues Maligna in an immunocompetent male: A case report. Journal of cosmetic dermatology. 2022 Jul:21(7):3160-3162. doi: 10.1111/jocd.15113. Epub 2022 Jun 4
[PubMed PMID: 35612931]
Level 3 (low-level) evidence
[101]
Wibisono O, Idrus I, Djawad K. Malignant Syphilis: A Systematic Review of the Case Reports Published in 2014-2018. Actas dermo-sifiliograficas. 2021 May 17:():. pii: S1578-2190(21)00174-8. doi: 10.1016/j.adengl.2021.05.014. Epub 2021 May 17
[PubMed PMID: 34052138]
Level 1 (high-level) evidence
[102]
Pradhan S, Sirka CS, Panda M, Baisakh M. Lues Maligna in an Immunocompetent Female. Indian dermatology online journal. 2018 Sep-Oct:9(5):344-346. doi: 10.4103/idoj.IDOJ_277_17. Epub
[PubMed PMID: 30258808]
[103]
Alves J, António AM, Matos D, Coelho R, Cachão P. Malignant lues in an immunocompetent patient. International journal of STD & AIDS. 2015 Jun:26(7):518-20. doi: 10.1177/0956462414544162. Epub 2014 Jul 11
[PubMed PMID: 25015934]
[104]
Young MF, Sanowski RA, Manne RA. Syphilitic hepatitis. Journal of clinical gastroenterology. 1992 Sep:15(2):174-6
[PubMed PMID: 1401840]
[105]
Plesa A, Gheorghe L, Hincu CE, Clim A, Nemteanu R. Making a Comeback: Syphilitic Hepatitis in a Patient with Late Latent Syphilis-Case Report and Review of the Literature. Pathogens (Basel, Switzerland). 2022 Oct 5:11(10):. doi: 10.3390/pathogens11101151. Epub 2022 Oct 5
[PubMed PMID: 36297208]
Level 3 (low-level) evidence
[106]
Ferrándiz-Pulido C, Ferrer B, Salcedo MT, Velasco M, Len O, Castells L. Secondary syphilis with liver involvement in a liver transplant recipient. Transplant infectious disease : an official journal of the Transplantation Society. 2021 Feb:23(1):e13431. doi: 10.1111/tid.13431. Epub 2020 Aug 16
[PubMed PMID: 32738832]
[107]
Traczuk A, Chetrit DA, Balasubramanya R, Nwaoduah N, Lee JB, Spacek LA, Loizidis G. Musculoskeletal manifestations of syphilis in adults: secondary syphilis presenting with ankle inflammatory arthritis and bone involvement with calvarial and sternal lesions. What the rheumatologist needs to know. Clinical rheumatology. 2023 Apr:42(4):1195-1203. doi: 10.1007/s10067-022-06458-8. Epub 2022 Dec 1
[PubMed PMID: 36454341]
[108]
Reginato AJ. Syphilitic arthritis and osteitis. Rheumatic diseases clinics of North America. 1993 May:19(2):379-98
[PubMed PMID: 8502778]
[109]
Hunte W, al-Ghraoui F, Cohen RJ. Secondary syphilis and the nephrotic syndrome. Journal of the American Society of Nephrology : JASN. 1993 Jan:3(7):1351-5
[PubMed PMID: 8439646]
[110]
Taniguchi M, Endo T, Asou M, Tsukamoto T. Nephrotic "full-house" glomerulonephritis successfully treated with antibiotics alone in secondary syphilis: a case report. CEN case reports. 2024 Apr:13(2):86-92. doi: 10.1007/s13730-023-00803-9. Epub 2023 Jun 23
[PubMed PMID: 37351772]
Level 3 (low-level) evidence
[111]
Shettigar R, Schollum J, Putt T, Chan L, Lau M, Walker R. Renal manifestations of syphilis. Internal medicine journal. 2021 Jul:51(7):1160-1167. doi: 10.1111/imj.15407. Epub
[PubMed PMID: 34278696]
[112]
Sciaudone M, Rutstein SE, Farel CE. Syphilis-Associated Acute Renal Failure and Hepatitis in the Setting of Human Immunodeficiency Virus Coinfection. Sexually transmitted diseases. 2019 Dec:46(12):816-818. doi: 10.1097/OLQ.0000000000001062. Epub
[PubMed PMID: 31764769]
[113]
Kennedy JL, Barnard JJ, Prahlow JA. Syphilitic coronary artery ostial stenosis resulting in acute myocardial infarction and death. Cardiology. 2006:105(1):25-9
[PubMed PMID: 16179782]
[114]
Miller SA, Ladich ER. Syphilitic Aortitis. The New England journal of medicine. 2022 May 19:386(20):e55. doi: 10.1056/NEJMicm2110835. Epub 2022 May 14
[PubMed PMID: 35550616]
[115]
Ip SKF, Lim K, Wong RHL. Syphilitic aortitis and ostial coronary stenosis treated by endarterectomy, bypass grafting and aortic valve replacement: a case report. AME case reports. 2023:7():15. doi: 10.21037/acr-22-32. Epub 2023 Feb 24
[PubMed PMID: 37122961]
Level 3 (low-level) evidence
[116]
Aizawa H, Hasegawa A, Arai M, Naganuma F, Hatori M, Kanda T, Suzuki T, Murata K, Satoh Y, Ishikawa S, Morishita Y, Nagai R. Bilateral coronary ostial stenosis and aortic regurgitation due to syphilitic aortitis. Internal medicine (Tokyo, Japan). 1998 Jan:37(1):56-9
[PubMed PMID: 9510401]
[117]
. Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 10-1998. A 46-year-old man with chest pain and coronary ostial stenosis. The New England journal of medicine. 1998 Mar 26:338(13):897-903
[PubMed PMID: 9518283]
Level 3 (low-level) evidence
[118]
Li X, Wang X, Wang Z, Du B, Mao C, Meng H, Meng F, Yang P. Cardiovascular syphilis-associated acute myocardial infarction: A case report. Medicine. 2021 Feb 19:100(7):e24788. doi: 10.1097/MD.0000000000024788. Epub
[PubMed PMID: 33607833]
Level 3 (low-level) evidence
[119]
Predescu LM, Zarma L, Platon P, Postu M, Bucsa A, Croitoru M, Prodan B, Chioncel O, Deleanu D. ST Segment Elevation Myocardial Infarction Due to Severe Ostial Left Main Stem Stenosis in a Patient with Syphilitic Aortitis. Romanian journal of internal medicine = Revue roumaine de medecine interne. 2016 Jan-Mar:54(1):74-9
[PubMed PMID: 27141575]
[120]
Nomura R, Yamazaki F, Egawa Y. Syphilitic aortitis: chronic left coronary ostial occlusion and aortic regurgitation with aortitis. General thoracic and cardiovascular surgery. 2021 Apr:69(4):736-739. doi: 10.1007/s11748-020-01523-y. Epub 2020 Oct 24
[PubMed PMID: 33098530]
[121]
Gayathri K, Shankar SV, Venkatesan S, Kalaivani S. Cardiovascular syphilis complicated by Lower thoracic and upper abdominal aneurysm - A rare case report. Indian journal of sexually transmitted diseases and AIDS. 2016 Jan-Jun:37(1):75-7. doi: 10.4103/0253-7184.180295. Epub
[PubMed PMID: 27190418]
Level 3 (low-level) evidence
[122]
Soares-Fernandes JP, Ribeiro M, Maré R, Magalhães Z, Lourenço E, Rocha JF. Diffusion-weighted magnetic resonance imaging findings in a patient with cerebral syphilitic gumma. Journal of computer assisted tomography. 2007 Jul-Aug:31(4):592-4
[PubMed PMID: 17882038]
[123]
Agrons GA, Han SS, Husson MA, Simeone F. MR imaging of cerebral gumma. AJNR. American journal of neuroradiology. 1991 Jan-Feb:12(1):80-1
[PubMed PMID: 1899524]
[124]
Li C, Wang SJ, Tang GC, Liu LT, Chen GX. Neuroimaging findings of cerebral syphilitic gumma. Experimental and therapeutic medicine. 2019 Dec:18(6):4185-4192. doi: 10.3892/etm.2019.8089. Epub 2019 Oct 8
[PubMed PMID: 31772624]
[125]
Moon J, Yu DA, Yoon HS, Cho S, Park HS. Syphilitic Gumma: A Rare Form of Cutaneous Tertiary Syphilis. Annals of dermatology. 2018 Dec:30(6):749-751. doi: 10.5021/ad.2018.30.6.749. Epub 2018 Oct 26
[PubMed PMID: 33911527]
[126]
Reschke R, Kunz M, Ziemer M. Gummatous Cutaneous Syphilis. Deutsches Arzteblatt international. 2022 Jul 1:119(26):457. doi: 10.3238/arztebl.m2022.0050. Epub
[PubMed PMID: 36321683]
[127]
Smullin C, Wagman J, Mehta S, Klausner JD. A Narrative Review of the Epidemiology of Congenital Syphilis in the United States From 1980 to 2019. Sexually transmitted diseases. 2021 Feb 1:48(2):71-78. doi: 10.1097/OLQ.0000000000001277. Epub
[PubMed PMID: 32925597]
Level 3 (low-level) evidence
[128]
The Lancet. Congenital syphilis in the USA. Lancet (London, England). 2018 Oct 6:392(10154):1168. doi: 10.1016/S0140-6736(18)32360-2. Epub
[PubMed PMID: 30319097]
[129]
Watson-Jones D, Changalucha J, Gumodoka B, Weiss H, Rusizoka M, Ndeki L, Whitehouse A, Balira R, Todd J, Ngeleja D, Ross D, Buvé A, Hayes R, Mabey D. Syphilis in pregnancy in Tanzania. I. Impact of maternal syphilis on outcome of pregnancy. The Journal of infectious diseases. 2002 Oct 1:186(7):940-7
[PubMed PMID: 12232834]
[131]
O'Connor NP, Gonzalez BE, Esper FP, Tamburro J, Kadkhoda K, Foster CB. Congenital syphilis: Missed opportunities and the case for rescreening during pregnancy and at delivery. IDCases. 2020:22():e00964. doi: 10.1016/j.idcr.2020.e00964. Epub 2020 Sep 23
[PubMed PMID: 33024697]
Level 3 (low-level) evidence
[132]
Mattei PL, Beachkofsky TM, Gilson RT, Wisco OJ. Syphilis: a reemerging infection. American family physician. 2012 Sep 1:86(5):433-40
[PubMed PMID: 22963062]
[133]
Kimball A, Bowen VB, Miele K, Weinstock H, Thorpe P, Bachmann L, McDonald R, Machefsky A, Torrone E. Congenital Syphilis Diagnosed Beyond the Neonatal Period in the United States: 2014-2018. Pediatrics. 2021 Sep:148(3):. doi: 10.1542/peds.2020-049080. Epub
[PubMed PMID: 34465590]
[134]
Almog B, Shehata F, Aljabri S, Levin I, Shalom-Paz E, Shrim A. Placenta weight percentile curves for singleton and twins deliveries. Placenta. 2011 Jan:32(1):58-62. doi: 10.1016/j.placenta.2010.10.008. Epub 2010 Oct 30
[PubMed PMID: 21036395]
[135]
Berman SM. Maternal syphilis: pathophysiology and treatment. Bulletin of the World Health Organization. 2004 Jun:82(6):433-8
[PubMed PMID: 15356936]
[136]
Genest DR, Choi-Hong SR, Tate JE, Qureshi F, Jacques SM, Crum C. Diagnosis of congenital syphilis from placental examination: comparison of histopathology, Steiner stain, and polymerase chain reaction for Treponema pallidum DNA. Human pathology. 1996 Apr:27(4):366-72
[PubMed PMID: 8617480]
[137]
Malan AF, Woods DL, Van der Elst CW, Meyer MP. Relative placental weight in congenital syphilis. Placenta. 1990 Jan-Feb:11(1):3-6
[PubMed PMID: 2326235]
[138]
Pillay S, Horn AR, Tooke L. Placental weights of neonates born with symptomatic congenital syphilis. Frontiers in pediatrics. 2023:11():1215387. doi: 10.3389/fped.2023.1215387. Epub 2023 Oct 6
[PubMed PMID: 37868268]
[139]
Stafford IA, Workowski KA, Bachmann LH. Syphilis Complicating Pregnancy and Congenital Syphilis. The New England journal of medicine. 2024 Jan 18:390(3):242-253. doi: 10.1056/NEJMra2202762. Epub
[PubMed PMID: 38231625]
[140]
David M, Hcini N, Mandelbrot L, Sibiude J, Picone O. Fetal and neonatal abnormalities due to congenital syphilis: A literature review. Prenatal diagnosis. 2022 May:42(5):643-655. doi: 10.1002/pd.6135. Epub 2022 Apr 9
[PubMed PMID: 35352829]
[141]
Workowski KA, Bolan GA, Centers for Disease Control and Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2015 Jun 5:64(RR-03):1-137
[PubMed PMID: 26042815]
[143]
Watson-Jones D, Gumodoka B, Weiss H, Changalucha J, Todd J, Mugeye K, Buvé A, Kanga Z, Ndeki L, Rusizoka M, Ross D, Marealle J, Balira R, Mabey D, Hayes R. Syphilis in pregnancy in Tanzania. II. The effectiveness of antenatal syphilis screening and single-dose benzathine penicillin treatment for the prevention of adverse pregnancy outcomes. The Journal of infectious diseases. 2002 Oct 1:186(7):948-57
[PubMed PMID: 12232835]
[144]
Dalby J, Stoner BP. Sexually Transmitted Infections: Updates From the 2021 CDC Guidelines. American family physician. 2022 May 1:105(5):514-520
[PubMed PMID: 35559639]
[145]
Watson-Jones D, Oliff M, Terris-Prestholt F, Changalucha J, Gumodoka B, Mayaud P, Semakafu AM, Kumaranayake L, Gavyole A, Mabey D, Hayes R. Antenatal syphilis screening in sub-Saharan Africa: lessons learned from Tanzania. Tropical medicine & international health : TM & IH. 2005 Sep:10(9):934-43
[PubMed PMID: 16135202]
[146]
Wendel GD Jr, Sheffield JS, Hollier LM, Hill JB, Ramsey PS, Sánchez PJ. Treatment of syphilis in pregnancy and prevention of congenital syphilis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Oct 15:35(Suppl 2):S200-9
[PubMed PMID: 12353207]
[147]
Zhu L, Qin M, Du L, Xie RH, Wong T, Wen SW. Maternal and congenital syphilis in Shanghai, China, 2002 to 2006. International journal of infectious diseases : IJID : official publication of the International Society for Infectious Diseases. 2010 Sep:14 Suppl 3():e45-8. doi: 10.1016/j.ijid.2009.09.009. Epub 2010 Feb 6
[PubMed PMID: 20137991]
Level 2 (mid-level) evidence
[148]
Ratnam S. The laboratory diagnosis of syphilis. The Canadian journal of infectious diseases & medical microbiology = Journal canadien des maladies infectieuses et de la microbiologie medicale. 2005 Jan:16(1):45-51
[PubMed PMID: 18159528]
[149]
Lejarraga-Cañas C, Ayerdi-Aguirrebengoa O, Menéndez-Prieto B, Tello-Romero E, Rodríguez-Martín C, Del Romero-Guerrero J. Is dark-field microscopy still useful for the primary syphilis diagnosis in the 21(ST) century? Enfermedades infecciosas y microbiologia clinica (English ed.). 2022 Jan:40(1):32-34. doi: 10.1016/j.eimce.2021.10.001. Epub 2021 Oct 31
[PubMed PMID: 34732343]
[150]
Janier M, Unemo M, Dupin N, Tiplica GS, Potočnik M, Patel R. 2020 European guideline on the management of syphilis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2021 Mar:35(3):574-588. doi: 10.1111/jdv.16946. Epub 2020 Oct 22
[PubMed PMID: 33094521]
[151]
Gayet-Ageron A, Combescure C, Lautenschlager S, Ninet B, Perneger TV. Comparison of Diagnostic Accuracy of PCR Targeting the 47-Kilodalton Protein Membrane Gene of Treponema pallidum and PCR Targeting the DNA Polymerase I Gene: Systematic Review and Meta-analysis. Journal of clinical microbiology. 2015 Nov:53(11):3522-9. doi: 10.1128/JCM.01619-15. Epub 2015 Aug 26
[PubMed PMID: 26311859]
Level 1 (high-level) evidence
[152]
Aung ET, Fairley CK, Williamson DA, Azzato F, Wigan R, Tran J, Buchanan A, Schmidt T, Chow EPF, Chen MY. Treponema pallidum PCR screening at mucosal sites of asymptomatic men who have sex with men taking HIV pre-exposure prophylaxis. Microbiology spectrum. 2023 Sep 6:11(5):e0079423. doi: 10.1128/spectrum.00794-23. Epub 2023 Sep 6
[PubMed PMID: 37671885]
[154]
Nayak S, Acharjya B. VDRL test and its interpretation. Indian journal of dermatology. 2012 Jan:57(1):3-8. doi: 10.4103/0019-5154.92666. Epub
[PubMed PMID: 22470199]
[155]
Janier M, Hegyi V, Dupin N, Unemo M, Tiplica GS, Potočnik M, French P, Patel R. 2014 European guideline on the management of syphilis. Journal of the European Academy of Dermatology and Venereology : JEADV. 2014 Dec:28(12):1581-93. doi: 10.1111/jdv.12734. Epub 2014 Oct 27
[PubMed PMID: 25348878]
[156]
Tuddenham S, Hamill MM, Ghanem KG. Diagnosis and Treatment of Sexually Transmitted Infections: A Review. JAMA. 2022 Jan 11:327(2):161-172. doi: 10.1001/jama.2021.23487. Epub
[PubMed PMID: 35015033]
[157]
Forrestel AK, Kovarik CL, Katz KA. Sexually acquired syphilis: Laboratory diagnosis, management, and prevention. Journal of the American Academy of Dermatology. 2020 Jan:82(1):17-28. doi: 10.1016/j.jaad.2019.02.074. Epub 2019 Apr 12
[PubMed PMID: 30986474]
[158]
Marra CM, Tantalo LC, Maxwell CL, Ho EL, Sahi SK, Jones T. The rapid plasma reagin test cannot replace the venereal disease research laboratory test for neurosyphilis diagnosis. Sexually transmitted diseases. 2012 Jun:39(6):453-7. doi: 10.1097/OLQ.0b013e31824b1cde. Epub
[PubMed PMID: 22592831]
[159]
Zondag HCA,van Dam AP,Bosch J,Getman D,Nenninger A,de Vries HJC,Bruisten SM, Timely diagnosis of incubating syphilis infections using Treponema pallidum Transcription Mediated Amplification assay. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2023 Aug 3;
[PubMed PMID: 37536265]
[160]
Getman D, Lin M, Barakat N, Skvoretz R, Godornes C, Swenson P, Nenninger A, Golden MR, Lukehart SA. Analytical Performance Characteristics of a New Transcription-Mediated Amplification Assay for Treponema pallidum. Journal of clinical microbiology. 2021 Jul 19:59(8):e0051121. doi: 10.1128/JCM.00511-21. Epub 2021 Jul 19
[PubMed PMID: 33980645]
[161]
Golden M, O'Donnell M, Lukehart S, Swenson P, Hovey P, Godornes C, Romano S, Getman D. Treponema pallidum Nucleic Acid Amplification Testing To Augment Syphilis Screening among Men Who Have Sex with Men. Journal of clinical microbiology. 2019 Aug:57(8):. doi: 10.1128/JCM.00572-19. Epub 2019 Jul 26
[PubMed PMID: 31189578]
[162]
Rodríguez I, Noda AA, Bosshard PP, Lienhard R. Anti-Treponema pallidum IgA response as a potential diagnostic marker of syphilis. Clinical microbiology and infection : the official publication of the European Society of Clinical Microbiology and Infectious Diseases. 2023 Dec:29(12):1603.e1-1603.e4. doi: 10.1016/j.cmi.2023.08.015. Epub 2023 Aug 21
[PubMed PMID: 37611864]
[163]
Cohen SE, Klausner JD, Engelman J, Philip S. Syphilis in the modern era: an update for physicians. Infectious disease clinics of North America. 2013 Dec:27(4):705-22. doi: 10.1016/j.idc.2013.08.005. Epub
[PubMed PMID: 24275265]
[164]
Amerson EH, Castillo Valladares HB, Leslie KS. Resurgence of Syphilis in the US-USPSTF Reaffirms Screening Guidelines. JAMA dermatology. 2022 Nov 1:158(11):1241-1243. doi: 10.1001/jamadermatol.2022.3499. Epub
[PubMed PMID: 36166232]
[165]
Morshed MG, Singh AE. Recent trends in the serologic diagnosis of syphilis. Clinical and vaccine immunology : CVI. 2015 Feb:22(2):137-47. doi: 10.1128/CVI.00681-14. Epub 2014 Nov 26
[PubMed PMID: 25428245]
[166]
Workowski KA, Bachmann LH, Chan PA, Johnston CM, Muzny CA, Park I, Reno H, Zenilman JM, Bolan GA. Sexually Transmitted Infections Treatment Guidelines, 2021. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2021 Jul 23:70(4):1-187. doi: 10.15585/mmwr.rr7004a1. Epub 2021 Jul 23
[PubMed PMID: 34292926]
[167]
Workowski KA, Bachmann LH. Centers for Disease Control and Prevention's Sexually Transmitted Diseases Infection Guidelines. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2022 Apr 13:74(74 Suppl 2):S89-S94. doi: 10.1093/cid/ciab1055. Epub
[PubMed PMID: 35416966]
[168]
Matsumoto H, Suzuki S, Nagata M, Senoo T, Watanabe M, Kawashima H. Clinical Features of Syphilis Patients with Ocular Symptoms as the Initial Manifestation. Clinical ophthalmology (Auckland, N.Z.). 2023:17():2901-2907. doi: 10.2147/OPTH.S425821. Epub 2023 Oct 3
[PubMed PMID: 37808000]
[169]
Hamill MM, Ghanem KG, Tuddenham S. State-of-the-Art Review: Neurosyphilis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2023 Aug 18:():. pii: ciad437. doi: 10.1093/cid/ciad437. Epub 2023 Aug 18
[PubMed PMID: 37593890]
[170]
Tuddenham S, Ghanem KG. Management of Adult Syphilis: Key Questions to Inform the 2021 Centers for Disease Control and Prevention Sexually Transmitted Infections Treatment Guidelines. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2022 Apr 13:74(Suppl_2):S127-S133. doi: 10.1093/cid/ciac060. Epub
[PubMed PMID: 35416969]
[171]
Libois A, De Wit S, Poll B, Garcia F, Florence E, Del Rio A, Sanchez P, Negredo E, Vandenbruaene M, Gatell JM, Clumeck N. HIV and syphilis: when to perform a lumbar puncture. Sexually transmitted diseases. 2007 Mar:34(3):141-4
[PubMed PMID: 16865051]
[172]
Ghanem KG, Moore RD, Rompalo AM, Erbelding EJ, Zenilman JM, Gebo KA. Lumbar puncture in HIV-infected patients with syphilis and no neurologic symptoms. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2009 Mar 15:48(6):816-21. doi: 10.1086/597096. Epub
[PubMed PMID: 19187028]
[173]
Cui W, Yan J, Weng W, Gao Y, Zhu W. Factors Associated With Neurosyphilis in Patients With Syphilis Treatment Failure: A Retrospective Study of 165 HIV-Negative Patients. Frontiers in medicine. 2022:9():757354. doi: 10.3389/fmed.2022.757354. Epub 2022 May 20
[PubMed PMID: 35669916]
Level 2 (mid-level) evidence
[174]
Wang YJ, Chi CY, Chou CH, Ho CM, Lin PC, Liao CH, Ho MW, Wang JH. Syphilis and neurosyphilis in human immunodeficiency virus-infected patients: a retrospective study at a teaching hospital in Taiwan. Journal of microbiology, immunology, and infection = Wei mian yu gan ran za zhi. 2012 Oct:45(5):337-42. doi: 10.1016/j.jmii.2011.12.011. Epub 2012 Jun 13
[PubMed PMID: 22698630]
Level 2 (mid-level) evidence
[175]
Castro R, Prieto ES, Aguas MJ, Manata MJ, Botas J, Araújo C, Borges F, Aldir I, Exposto Fda L. Evaluation of the Treponema pallidum particle agglutination technique (TP.PA) in the diagnosis of neurosyphilis. Journal of clinical laboratory analysis. 2006:20(6):233-8
[PubMed PMID: 17115423]
[176]
Ding D, Gao J, Zhang W, Xu D. The Diagnostic Performance of Laboratory Tests of Neurosyphilis: A Systematic Review and Network Meta-Analysis. European neurology. 2023:86(6):418-429. doi: 10.1159/000531341. Epub 2023 Aug 7
[PubMed PMID: 37549649]
Level 1 (high-level) evidence
[177]
Yang L, Fu Y, Li S, Liu C, Liu D. Analysis of Treponema pallidum DNA and CXCL13 in Cerebrospinal Fluid in HIV-Negative Syphilis Patients. Infection and drug resistance. 2022:15():7791-7798. doi: 10.2147/IDR.S394581. Epub 2022 Dec 29
[PubMed PMID: 36600952]
[179]
Papp JR, Park IU, Fakile Y, Pereira L, Pillay A, Bolan GA. CDC Laboratory Recommendations for Syphilis Testing, United States, 2024. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2024 Feb 8:73(1):1-32. doi: 10.15585/mmwr.rr7301a1. Epub 2024 Feb 8
[PubMed PMID: 38319847]
[180]
Marra CM, Critchlow CW, Hook EW 3rd, Collier AC, Lukehart SA. Cerebrospinal fluid treponemal antibodies in untreated early syphilis. Archives of neurology. 1995 Jan:52(1):68-72
[PubMed PMID: 7826278]
[181]
Berger JR. Neurosyphilis and the spinal cord: then and now. The Journal of nervous and mental disease. 2011 Dec:199(12):912-3. doi: 10.1097/NMD.0b013e31823928e8. Epub
[PubMed PMID: 22134447]
[183]
Guarner J, Jost H, Pillay A, Sun Y, Cox D, Notenboom R, Workowski K. Evaluation of treponemal serum tests performed on cerebrospinal fluid for diagnosis of neurosyphilis. American journal of clinical pathology. 2015 Apr:143(4):479-84. doi: 10.1309/AJCPWSL3G8RXMCQR. Epub
[PubMed PMID: 25779998]
[184]
Marra CM, Tantalo LC, Maxwell CL, Dougherty K, Wood B. Alternative cerebrospinal fluid tests to diagnose neurosyphilis in HIV-infected individuals. Neurology. 2004 Jul 13:63(1):85-8
[PubMed PMID: 15249615]
[185]
Luger A, Schmidt BL, Steyrer K, Schonwald E. Diagnosis of neurosyphilis by examination of the cerebrospinal fluid. The British journal of venereal diseases. 1981 Aug:57(4):232-7
[PubMed PMID: 7023601]
[186]
Tagarro A, García-Alix A, Alarcón A, Hernanz A, Quero J. Congenital syphilis: beta2-microglobulin in cerebrospinal fluid and diagnosis of neurosyphilis in an affected newborn. Journal of perinatal medicine. 2005:33(1):79-82
[PubMed PMID: 15841621]
[187]
Marra CM, Tantalo LC, Sahi SK, Maxwell CL, Lukehart SA. CXCL13 as a cerebrospinal fluid marker for neurosyphilis in HIV-infected patients with syphilis. Sexually transmitted diseases. 2010 May:37(5):283-7. doi: 10.1097/OLQ.0b013e3181d877a1. Epub
[PubMed PMID: 20393380]
[188]
Khamaysi Z, Bergman R, Telman G, Goldsher D. Clinical and imaging findings in patients with neurosyphilis: a study of a cohort and review of the literature. International journal of dermatology. 2014 Jul:53(7):812-9. doi: 10.1111/ijd.12095. Epub 2013 Nov 21
[PubMed PMID: 24261864]
[189]
Nagappa M, Sinha S, Taly AB, Rao SL, Nagarathna S, Bindu PS, Bharath RD, Murthy P. Neurosyphilis: MRI features and their phenotypic correlation in a cohort of 35 patients from a tertiary care university hospital. Neuroradiology. 2013 Mar:55(4):379-88. doi: 10.1007/s00234-012-1017-9. Epub 2012 Dec 30
[PubMed PMID: 23274762]
[190]
Corrêa DG, de Souza SR, Freddi TAL, Fonseca APA, Dos Santos RQ, Hygino da Cruz LC Jr. Imaging features of neurosyphilis. Journal of neuroradiology = Journal de neuroradiologie. 2023 Mar:50(2):241-252. doi: 10.1016/j.neurad.2023.01.003. Epub 2023 Jan 11
[PubMed PMID: 36641134]
[191]
Toskin I, Peeling RW, Mabey D, Holmes K, Ballard R, Kiarie J, Askew I. Point-of-care tests for STIs: the way forward. Sexually transmitted infections. 2017 Dec:93(S4):S1-S2. doi: 10.1136/sextrans-2016-053074. Epub
[PubMed PMID: 29223958]
[192]
Nakku-Joloba E, Kiragga A, Mbazira JK, Kambugu F, Jett-Goheen M, Ratanshi RP, Gaydos C, Manabe YC. Clinical Evaluation of 2 Point-of-Care Lateral Flow Tests for the Diagnosis of Syphilis. Sexually transmitted diseases. 2016 Oct:43(10):623-5. doi: 10.1097/OLQ.0000000000000498. Epub
[PubMed PMID: 27631356]
[193]
Herring A, Ballard R, Mabey D, Peeling RW, WHO/TDR Sexually Transmitted Diseases Diagnostics Initiative. Evaluation of rapid diagnostic tests: syphilis. Nature reviews. Microbiology. 2006 Dec:4(12 Suppl):S33-40
[PubMed PMID: 17366685]
[194]
Tucker JD, Bu J, Brown LB, Yin YP, Chen XS, Cohen MS. Accelerating worldwide syphilis screening through rapid testing: a systematic review. The Lancet. Infectious diseases. 2010 Jun:10(6):381-6. doi: 10.1016/S1473-3099(10)70092-X. Epub
[PubMed PMID: 20510278]
Level 1 (high-level) evidence
[195]
Jafari Y, Peeling RW, Shivkumar S, Claessens C, Joseph L, Pai NP. Are Treponema pallidum specific rapid and point-of-care tests for syphilis accurate enough for screening in resource limited settings? Evidence from a meta-analysis. PloS one. 2013:8(2):e54695. doi: 10.1371/journal.pone.0054695. Epub 2013 Feb 26
[PubMed PMID: 23468842]
Level 1 (high-level) evidence
[196]
Naidu P, Tsang RS. Canadian Public Health Laboratory Network guidelines for the use of point-of-care tests for Treponema pallidum in Canada. Journal of the Association of Medical Microbiology and Infectious Disease Canada = Journal officiel de l'Association pour la microbiologie medicale et l'infectiologie Canada. 2022 Jun:7(2):85-96. doi: 10.3138/jammi-2021-0021. Epub 2022 Jun 3
[PubMed PMID: 36337357]
[197]
Taylor MM, Peeling RW, Toskin I, Ghinidelli M. Role of dual HIV/syphilis test kits in expanding syphilis screening. Sexually transmitted infections. 2017 Nov:93(7):458-459. doi: 10.1136/sextrans-2017-053301. Epub 2017 Aug 4
[PubMed PMID: 28778981]
[198]
Gliddon HD, Peeling RW, Kamb ML, Toskin I, Wi TE, Taylor MM. A systematic review and meta-analysis of studies evaluating the performance and operational characteristics of dual point-of-care tests for HIV and syphilis. Sexually transmitted infections. 2017 Dec:93(S4):S3-S15. doi: 10.1136/sextrans-2016-053069. Epub 2017 Jul 26
[PubMed PMID: 28747410]
Level 1 (high-level) evidence
[199]
Pham MD, Ong JJ, Anderson DA, Drummer HE, Stoové M. Point-of-Care Diagnostics for Diagnosis of Active Syphilis Infection: Needs, Challenges and the Way Forward. International journal of environmental research and public health. 2022 Jul 4:19(13):. doi: 10.3390/ijerph19138172. Epub 2022 Jul 4
[PubMed PMID: 35805831]
[200]
Pereira LE, McCormick J, Dorji T, Kang J, Sun Y, Shukla M, Hopkins A, Deutsch J, Kersh EN, Bernstein K, Fakile YF. Laboratory Evaluation of a Commercially Available Rapid Syphilis Test. Journal of clinical microbiology. 2018 Oct:56(10):. doi: 10.1128/JCM.00832-18. Epub 2018 Sep 25
[PubMed PMID: 30021825]
[201]
Peterman TA, Fakile YF. What Is the Use of Rapid Syphilis Tests in the United States? Sexually transmitted diseases. 2016 Mar:43(3):201-3. doi: 10.1097/OLQ.0000000000000413. Epub
[PubMed PMID: 26859809]
[202]
Swartzendruber A, Steiner RJ, Adler MR, Kamb ML, Newman LM. Introduction of rapid syphilis testing in antenatal care: A systematic review of the impact on HIV and syphilis testing uptake and coverage. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2015 Jun:130 Suppl 1(Suppl 1):S15-21. doi: 10.1016/j.ijgo.2015.04.008. Epub 2015 Apr 29
[PubMed PMID: 26001704]
Level 1 (high-level) evidence
[203]
Towns JM, Tieosapjaroen W, Mello MB, Baggaley RC, Johnson CC, Jamil MS, Rowley J, Barr-DiChiara M, Terris-Prestholt F, Chen MY, Chow EPF, Fairley CK, Zhang L, Ong JJ. The role of syphilis self-testing as an additional syphilis testing approach in key populations: a systematic review and meta-analysis. The Lancet. Public health. 2023 Sep:8(9):e726-e734. doi: 10.1016/S2468-2667(23)00128-7. Epub 2023 Jul 21
[PubMed PMID: 37482070]
Level 1 (high-level) evidence
[204]
Mabey DC, Sollis KA, Kelly HA, Benzaken AS, Bitarakwate E, Changalucha J, Chen XS, Yin YP, Garcia PJ, Strasser S, Chintu N, Pang T, Terris-Prestholt F, Sweeney S, Peeling RW. Point-of-care tests to strengthen health systems and save newborn lives: the case of syphilis. PLoS medicine. 2012:9(6):e1001233. doi: 10.1371/journal.pmed.1001233. Epub 2012 Jun 12
[PubMed PMID: 22719229]
Level 3 (low-level) evidence
[205]
Ribeiro LV, Sabidó M, Galbán E, Guerra JA, Mabey D, Peeling RW, Benzaken AS. Home-based counseling and testing for HIV and syphilis - an evaluation of acceptability and quality control, in remote Amazonas State, Brazil. Sexually transmitted infections. 2015 Mar:91(2):94-6. doi: 10.1136/sextrans-2014-051625. Epub 2014 Oct 10
[PubMed PMID: 25305212]
Level 2 (mid-level) evidence
[206]
García PJ, Cárcamo CP, Chiappe M, Valderrama M, La Rosa S, Holmes KK, Mabey DC, Peeling RW. Rapid Syphilis Tests as Catalysts for Health Systems Strengthening: A Case Study from Peru. PloS one. 2013:8(6):e66905. doi: 10.1371/journal.pone.0066905. Epub 2013 Jun 26
[PubMed PMID: 23840552]
Level 3 (low-level) evidence
[207]
Chitneni P, Owembabazi M, Muyindike W, Asiimwe S, Masete G, Mbalibulha Y, Nakku-Joloba E, Manabe YC, Haberer JE, Matthews LT, Van Der Pol B. Sexually Transmitted Infection Point-of-Care Testing in Resource-Limited Settings: A Narrative Review Guided by an Implementation Framework. Sexually transmitted diseases. 2023 Oct 1:50(10):e11-e16. doi: 10.1097/OLQ.0000000000001848. Epub 2023 Jul 4
[PubMed PMID: 37433000]
Level 3 (low-level) evidence
[209]
Shockman S, Buescher LS, Stone SP. Syphilis in the United States. Clinics in dermatology. 2014 Mar-Apr:32(2):213-8. doi: 10.1016/j.clindermatol.2013.08.005. Epub
[PubMed PMID: 24559556]
[210]
Sullins AK,Abdel-Rahman SM, Pharmacokinetics of antibacterial agents in the CSF of children and adolescents. Paediatric drugs. 2013 Apr
[PubMed PMID: 23529866]
[212]
Fisher JF, Mobashery S. Constructing and deconstructing the bacterial cell wall. Protein science : a publication of the Protein Society. 2020 Mar:29(3):629-646. doi: 10.1002/pro.3737. Epub 2019 Nov 20
[PubMed PMID: 31747090]
[213]
Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annual review of microbiology. 1979:33():113-37
[PubMed PMID: 40528]
[214]
Park JT, Uehara T. How bacteria consume their own exoskeletons (turnover and recycling of cell wall peptidoglycan). Microbiology and molecular biology reviews : MMBR. 2008 Jun:72(2):211-27, table of contents. doi: 10.1128/MMBR.00027-07. Epub
[PubMed PMID: 18535144]
[215]
Wise EM Jr, Park JT. Penicillin: its basic site of action as an inhibitor of a peptide cross-linking reaction in cell wall mucopeptide synthesis. Proceedings of the National Academy of Sciences of the United States of America. 1965 Jul:54(1):75-81
[PubMed PMID: 5216369]
[216]
Tipper DJ, Strominger JL. Mechanism of action of penicillins: a proposal based on their structural similarity to acyl-D-alanyl-D-alanine. Proceedings of the National Academy of Sciences of the United States of America. 1965 Oct:54(4):1133-41
[PubMed PMID: 5219821]
[217]
Josephine HR, Kumar I, Pratt RF. The perfect penicillin? Inhibition of a bacterial DD-peptidase by peptidoglycan-mimetic beta-lactams. Journal of the American Chemical Society. 2004 Jul 7:126(26):8122-3
[PubMed PMID: 15225046]
[218]
Kohanski MA, Dwyer DJ, Collins JJ. How antibiotics kill bacteria: from targets to networks. Nature reviews. Microbiology. 2010 Jun:8(6):423-35. doi: 10.1038/nrmicro2333. Epub 2010 May 4
[PubMed PMID: 20440275]
[219]
Collart P, Poitevin M, Milovanovic A, Herlin A, Durel J. Kinetic study of serum penicillin concentrations after single doses of benzathine and benethamine penicillins in young and old people. The British journal of venereal diseases. 1980 Dec:56(6):355-62
[PubMed PMID: 7448577]
[221]
Xiao Y, Tong ML, Lin LR, Liu LL, Gao K, Chen MJ, Zhang HL, Zheng WH, Li SL, Lin HL, Lin ZF, Yang TC, Niu JJ. Serological Response Predicts Normalization of Cerebrospinal Fluid Abnormalities at Six Months after Treatment in HIV-Negative Neurosyphilis Patients. Scientific reports. 2017 Aug 30:7(1):9911. doi: 10.1038/s41598-017-10387-x. Epub 2017 Aug 30
[PubMed PMID: 28855625]
[222]
Marra CM, Maxwell CL, Tantalo LC, Sahi SK, Lukehart SA. Normalization of serum rapid plasma reagin titer predicts normalization of cerebrospinal fluid and clinical abnormalities after treatment of neurosyphilis. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2008 Oct 1:47(7):893-9. doi: 10.1086/591534. Epub
[PubMed PMID: 18715154]
[223]
Salle R, Grange PA, Ollagnier G, Hembert R, Benhaddou N, Heller U, Dupin N. Treponema pallidum resistance to azithromycin in France: A nationwide retrospective study from 2010 to 2022. Journal of the European Academy of Dermatology and Venereology : JEADV. 2024 Jan:38(1):e20-e21. doi: 10.1111/jdv.19399. Epub 2023 Aug 19
[PubMed PMID: 37561930]
Level 2 (mid-level) evidence
[224]
Venter JME, Müller EE, Mahlangu MP, Kularatne RS. Treponema pallidum Macrolide Resistance and Molecular Epidemiology in Southern Africa, 2008 to 2018. Journal of clinical microbiology. 2021 Sep 20:59(10):e0238520. doi: 10.1128/JCM.02385-20. Epub 2021 Aug 4
[PubMed PMID: 34346717]
[225]
Eikmeier D, Talley P, Bowen A, Leano F, Dobbins G, Jawahir S, Gross A, Huspeni D, La Pointe A, Meyer S, Smith K. Decreased Susceptibility to Azithromycin in Clinical Shigella Isolates Associated with HIV and Sexually Transmitted Bacterial Diseases, Minnesota, USA, 2012-2015. Emerging infectious diseases. 2020 Apr:26(4):667-674. doi: 10.3201/eid2604.191031. Epub
[PubMed PMID: 32186495]
[226]
Lukehart SA, Godornes C, Molini BJ, Sonnett P, Hopkins S, Mulcahy F, Engelman J, Mitchell SJ, Rompalo AM, Marra CM, Klausner JD. Macrolide resistance in Treponema pallidum in the United States and Ireland. The New England journal of medicine. 2004 Jul 8:351(2):154-8
[PubMed PMID: 15247355]
[227]
Holmes KK. Azithromycin versus penicillin G benzathine for early syphilis. The New England journal of medicine. 2005 Sep 22:353(12):1291-3
[PubMed PMID: 16177256]
[228]
Katz KA, Klausner JD. Azithromycin resistance in Treponema pallidum. Current opinion in infectious diseases. 2008 Feb:21(1):83-91. doi: 10.1097/QCO.0b013e3282f44772. Epub
[PubMed PMID: 18192791]
Level 3 (low-level) evidence
[229]
Tipple C, Taylor GP. Syphilis testing, typing, and treatment follow-up: a new era for an old disease. Current opinion in infectious diseases. 2015 Feb:28(1):53-60. doi: 10.1097/QCO.0000000000000124. Epub
[PubMed PMID: 25485649]
Level 3 (low-level) evidence
[230]
Clement ME, Okeke NL, Hicks CB. Treatment of syphilis: a systematic review. JAMA. 2014 Nov 12:312(18):1905-17. doi: 10.1001/jama.2014.13259. Epub
[PubMed PMID: 25387188]
Level 1 (high-level) evidence
[232]
Haynes AM, Giacani L, Mayans MV, Ubals M, Nieto C, Pérez-Mañá C, Quintó L, Romeis E, Mitjà O. Efficacy of linezolid on Treponema pallidum, the syphilis agent: A preclinical study. EBioMedicine. 2021 Mar:65():103281. doi: 10.1016/j.ebiom.2021.103281. Epub 2021 Mar 12
[PubMed PMID: 33721817]
[233]
Dryden MS. Linezolid pharmacokinetics and pharmacodynamics in clinical treatment. The Journal of antimicrobial chemotherapy. 2011 May:66 Suppl 4():iv7-iv15. doi: 10.1093/jac/dkr072. Epub
[PubMed PMID: 21521707]
[234]
Metaxas EI, Falagas ME. Update on the safety of linezolid. Expert opinion on drug safety. 2009 Jul:8(4):485-91. doi: 10.1517/14740330903049706. Epub
[PubMed PMID: 19538105]
Level 3 (low-level) evidence
[235]
U.S. Preventive Services Task Force. Screening for syphilis infection in pregnancy: U.S. Preventive Services Task Force reaffirmation recommendation statement. Annals of internal medicine. 2009 May 19:150(10):705-9
[PubMed PMID: 19451577]
[236]
Hawkes S, Matin N, Broutet N, Low N. Effectiveness of interventions to improve screening for syphilis in pregnancy: a systematic review and meta-analysis. The Lancet. Infectious diseases. 2011 Sep:11(9):684-91. doi: 10.1016/S1473-3099(11)70104-9. Epub 2011 Jun 15
[PubMed PMID: 21683653]
Level 1 (high-level) evidence
[238]
Terris-Prestholt F, Watson-Jones D, Mugeye K, Kumaranayake L, Ndeki L, Weiss H, Changalucha J, Todd J, Lisekie F, Gumodoka B, Mabey D, Hayes R. Is antenatal syphilis screening still cost effective in sub-Saharan Africa. Sexually transmitted infections. 2003 Oct:79(5):375-81
[PubMed PMID: 14573832]
[239]
Terris-Prestholt F, Vickerman P, Torres-Rueda S, Santesso N, Sweeney S, Mallma P, Shelley KD, Garcia PJ, Bronzan R, Gill MM, Broutet N, Wi T, Watts C, Mabey D, Peeling RW, Newman L. The cost-effectiveness of 10 antenatal syphilis screening and treatment approaches in Peru, Tanzania, and Zambia. International journal of gynaecology and obstetrics: the official organ of the International Federation of Gynaecology and Obstetrics. 2015 Jun:130 Suppl 1(Suppl 1):S73-80. doi: 10.1016/j.ijgo.2015.04.007. Epub 2015 Apr 29
[PubMed PMID: 25963907]
[240]
Hollier LM, Harstad TW, Sanchez PJ, Twickler DM, Wendel GD Jr. Fetal syphilis: clinical and laboratory characteristics. Obstetrics and gynecology. 2001 Jun:97(6):947-53
[PubMed PMID: 11384701]
[241]
Rosenthal M, Poliquin V. Exploring management of antenatally diagnosed fetal syphilis infection. Canada communicable disease report = Releve des maladies transmissibles au Canada. 2022 Feb 24:48(2-3):111-114. doi: 10.14745/ccdr.v48i23a09. Epub 2022 Feb 24
[PubMed PMID: 35342369]
[242]
Rac MW, Bryant SN, Cantey JB, McIntire DD, Wendel GD Jr, Sheffield JS. Maternal titers after adequate syphilotherapy during pregnancy. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Mar 1:60(5):686-90. doi: 10.1093/cid/ciu920. Epub 2014 Nov 19
[PubMed PMID: 25414264]
[243]
Pham MN, Ho HE, Desai M. Penicillin desensitization: Treatment of syphilis in pregnancy in penicillin-allergic patients. Annals of allergy, asthma & immunology : official publication of the American College of Allergy, Asthma, & Immunology. 2017 May:118(5):537-541. doi: 10.1016/j.anai.2017.03.013. Epub
[PubMed PMID: 28477786]
[245]
Shann S, Wilson J. Treatment of neurosyphilis with ceftriaxone. Sexually transmitted infections. 2003 Oct:79(5):415-6
[PubMed PMID: 14573840]
[246]
Felix MMR, Aun MV, Menezes UP, Queiroz GRES, Rodrigues AT, D'Onofrio-Silva AC, Perelló MI, Camelo-Nunes IC, Malaman MF. Allergy to penicillin and betalactam antibiotics. Einstein (Sao Paulo, Brazil). 2021:19():eMD5703. doi: 10.31744/einstein_journal/2021MD5703. Epub 2021 Apr 26
[PubMed PMID: 33909756]
[247]
Trubiano JA, Thursky KA, Stewardson AJ, Urbancic K, Worth LJ, Jackson C, Stevenson W, Sutherland M, Slavin MA, Grayson ML, Phillips EJ. Impact of an Integrated Antibiotic Allergy Testing Program on Antimicrobial Stewardship: A Multicenter Evaluation. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Jul 1:65(1):166-174. doi: 10.1093/cid/cix244. Epub
[PubMed PMID: 28520865]
[248]
Chen JR, Tarver SA, Alvarez KS, Tran T, Khan DA. A Proactive Approach to Penicillin Allergy Testing in Hospitalized Patients. The journal of allergy and clinical immunology. In practice. 2017 May-Jun:5(3):686-693. doi: 10.1016/j.jaip.2016.09.045. Epub 2016 Nov 23
[PubMed PMID: 27888034]
[249]
Shenoy ES, Macy E, Rowe T, Blumenthal KG. Evaluation and Management of Penicillin Allergy: A Review. JAMA. 2019 Jan 15:321(2):188-199. doi: 10.1001/jama.2018.19283. Epub
[PubMed PMID: 30644987]
[250]
Bettuzzi T, Jourdes A, Robineau O, Alcaraz I, Manda V, Molina JM, Mehlen M, Cazanave C, Tattevin P, Mensi S, Terrier B, Régent A, Ghosn J, Charlier C, Martin-Blondel G, Dupin N. Ceftriaxone compared with benzylpenicillin in the treatment of neurosyphilis in France: a retrospective multicentre study. The Lancet. Infectious diseases. 2021 Oct:21(10):1441-1447. doi: 10.1016/S1473-3099(20)30857-4. Epub 2021 May 26
[PubMed PMID: 34051142]
Level 2 (mid-level) evidence
[252]
Schöfer H, Vogt HJ, Milbradt R. Ceftriaxone for the treatment of primary and secondary syphilis. Chemotherapy. 1989:35(2):140-5
[PubMed PMID: 2569381]
[253]
Cao Y, Su X, Wang Q, Xue H, Zhu X, Zhang C, Jiang J, Qi S, Gong X, Zhu X, Pan M, Ren H, Hu W, Wei Z, Tian M, Liu W. A Multicenter Study Evaluating Ceftriaxone and Benzathine Penicillin G as Treatment Agents for Early Syphilis in Jiangsu, China. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2017 Oct 30:65(10):1683-1688. doi: 10.1093/cid/cix611. Epub
[PubMed PMID: 29020150]
Level 2 (mid-level) evidence
[254]
Lang R, Shalit I, Segal J, Arbel Y, Markov S, Hass H, Fejgin M. Maternal and fetal serum and tissue levels of ceftriaxone following preoperative prophylaxis in emergency cesarean section. Chemotherapy. 1993 Mar-Apr:39(2):77-81
[PubMed PMID: 8458249]
[255]
Bourget P, Fernandez H, Quinquis V, Delouis C. Pharmacokinetics and protein binding of ceftriaxone during pregnancy. Antimicrobial agents and chemotherapy. 1993 Jan:37(1):54-9
[PubMed PMID: 8431018]
[256]
Patel IH, Kaplan SA. Pharmacokinetic profile of ceftriaxone in man. The American journal of medicine. 1984 Oct 19:77(4C):17-25
[PubMed PMID: 6093513]
[257]
Coyle M, Depcinski S, Thirumoorthi M. Prevention of congenital syphilis using ceftriaxone in a woman with Stevens-Johnson syndrome reaction to penicillin: A case report. Case reports in women's health. 2022 Oct:36():e00446. doi: 10.1016/j.crwh.2022.e00446. Epub 2022 Aug 20
[PubMed PMID: 36072694]
Level 3 (low-level) evidence
[258]
Garcia JFB, Aun MV, Motta AA, Castells M, Kalil J, Giavina-Bianchi P. Algorithm to guide re-exposure to penicillin in allergic pregnant women with syphilis: Efficacy and safety. The World Allergy Organization journal. 2021 Jun:14(6):100549. doi: 10.1016/j.waojou.2021.100549. Epub 2021 May 21
[PubMed PMID: 34093957]
[259]
Gill MM, Gasner S, Banken A, Park M, Weaver A, Sharpe E, Theiler R. Improving routine prenatal penicillin allergy testing for reported penicillin allergy. BMJ open quality. 2022 Jul:11(3):. doi: 10.1136/bmjoq-2022-001859. Epub
[PubMed PMID: 35906008]
Level 2 (mid-level) evidence
[260]
Thellier C, Subtil D, Pelletier de Chambure D, Grandbastien B, Catteau C, Beaugendre A, Poitrenaud D, Prevotat A, Richart P, Faure K, Le Guern R. An educational intervention about the classification of penicillin allergies: effect on the appropriate choice of antibiotic therapy in pregnant women. International journal of obstetric anesthesia. 2020 Feb:41():22-28. doi: 10.1016/j.ijoa.2019.07.005. Epub 2019 Jul 18
[PubMed PMID: 31402310]
[262]
Tran NK, Goldstein ND, Welles SL. Countering the rise of syphilis: A role for doxycycline post-exposure prophylaxis? International journal of STD & AIDS. 2022 Jan:33(1):18-30. doi: 10.1177/09564624211042444. Epub 2021 Sep 26
[PubMed PMID: 34565255]
[263]
Luetkemeyer AF, Donnell D, Dombrowski JC, Cohen S, Grabow C, Brown CE, Malinski C, Perkins R, Nasser M, Lopez C, Vittinghoff E, Buchbinder SP, Scott H, Charlebois ED, Havlir DV, Soge OO, Celum C, DoxyPEP Study Team. Postexposure Doxycycline to Prevent Bacterial Sexually Transmitted Infections. The New England journal of medicine. 2023 Apr 6:388(14):1296-1306. doi: 10.1056/NEJMoa2211934. Epub
[PubMed PMID: 37018493]
[264]
Melendez JH, Hamill MM, Armington GS, Gaydos CA, Manabe YC. Home-Based Testing for Sexually Transmitted Infections: Leveraging Online Resources During the COVID-19 Pandemic. Sexually transmitted diseases. 2021 Jan:48(1):e8-e10. doi: 10.1097/OLQ.0000000000001309. Epub
[PubMed PMID: 33229964]
[265]
Odesanmi TY, Wasti SP, Odesanmi OS, Adegbola O, Oguntuase OO, Mahmood S. Comparative effectiveness and acceptability of home-based and clinic-based sampling methods for sexually transmissible infections screening in females aged 14-50 years: a systematic review and meta-analysis. Sexual health. 2013 Dec:10(6):559-69. doi: 10.1071/SH13029. Epub
[PubMed PMID: 24160747]
Level 1 (high-level) evidence
[266]
Amador-Fernández N, Gastelurrutia MÁ, García-Cárdenas V. Development of self-care in Spanish community pharmacies. Exploratory research in clinical and social pharmacy. 2023 Dec:12():100337. doi: 10.1016/j.rcsop.2023.100337. Epub 2023 Sep 26
[PubMed PMID: 37841580]
[267]
Shih SL, Graseck AS, Secura GM, Peipert JF. Screening for sexually transmitted infections at home or in the clinic? Current opinion in infectious diseases. 2011 Feb:24(1):78-84. doi: 10.1097/QCO.0b013e32834204a8. Epub
[PubMed PMID: 21124216]
Level 3 (low-level) evidence
[268]
Leenen J, Hoebe CJPA, Ackens RP, Posthouwer D, van Loo IHM, Wolffs PFG, Dukers-Muijrers NHTM. Pilot implementation of a home-care programme with chlamydia, gonorrhoea, hepatitis B, and syphilis self-sampling in HIV-positive men who have sex with men. BMC infectious diseases. 2020 Dec 4:20(1):925. doi: 10.1186/s12879-020-05658-4. Epub 2020 Dec 4
[PubMed PMID: 33276727]
Level 3 (low-level) evidence
[269]
van Loo IHM, Dukers-Muijrers NHTM, Heuts R, van der Sande MAB, Hoebe CJPA. Screening for HIV, hepatitis B and syphilis on dried blood spots: A promising method to better reach hidden high-risk populations with self-collected sampling. PloS one. 2017:12(10):e0186722. doi: 10.1371/journal.pone.0186722. Epub 2017 Oct 20
[PubMed PMID: 29053737]
[270]
Siguier M, Molina JM. Doxycycline Prophylaxis for Bacterial Sexually Transmitted Infections: Promises and Perils. ACS infectious diseases. 2018 May 11:4(5):660-663. doi: 10.1021/acsinfecdis.8b00043. Epub 2018 Mar 23
[PubMed PMID: 29570279]
[271]
Ávila-Nieto C, Pedreño-López N, Mitjà O, Clotet B, Blanco J, Carrillo J. Syphilis vaccine: challenges, controversies and opportunities. Frontiers in immunology. 2023:14():1126170. doi: 10.3389/fimmu.2023.1126170. Epub 2023 Apr 6
[PubMed PMID: 37090699]
[272]
Gottlieb SL, Deal CD, Giersing B, Rees H, Bolan G, Johnston C, Timms P, Gray-Owen SD, Jerse AE, Cameron CE, Moorthy VS, Kiarie J, Broutet N. The global roadmap for advancing development of vaccines against sexually transmitted infections: Update and next steps. Vaccine. 2016 Jun 3:34(26):2939-2947. doi: 10.1016/j.vaccine.2016.03.111. Epub 2016 Apr 19
[PubMed PMID: 27105564]
[273]
Kojima N, Konda KA, Klausner JD. Notes on syphilis vaccine development. Frontiers in immunology. 2022:13():952284. doi: 10.3389/fimmu.2022.952284. Epub 2022 Jul 28
[PubMed PMID: 35967432]
[274]
Lukehart SA, Molini B, Gomez A, Godornes C, Hof R, Fernandez MC, Pitner RA, Gray SA, Carter D, Giacani L, Cameron CE. Immunization with a tri-antigen syphilis vaccine significantly attenuates chancre development, reduces bacterial load, and inhibits dissemination of Treponema pallidum. Vaccine. 2022 Dec 12:40(52):7676-7692. doi: 10.1016/j.vaccine.2022.11.002. Epub 2022 Nov 12
[PubMed PMID: 36376214]
[275]
Li S, Li W, Jin Y, Wu B, Wu Y. Advancements in the development of nucleic acid vaccines for syphilis prevention and control. Human vaccines & immunotherapeutics. 2023 Aug 1:19(2):2234790. doi: 10.1080/21645515.2023.2234790. Epub
[PubMed PMID: 37538024]
[276]
Chen J, Huang J, Liu Z, Xie Y. Treponema pallidum outer membrane proteins: current status and prospects. Pathogens and disease. 2022 Sep 27:80(1):. pii: ftac023. doi: 10.1093/femspd/ftac023. Epub
[PubMed PMID: 35869970]
[277]
Shukla MR, Pereira L, Gaynor AM, Sun Y, Edwards D, Simmons T, Andrews CW, Park IU, Hong J, Cao W, Kersh EN, Fakile Y. Evaluation of Three Automated Nontreponemal Rapid Plasma Reagin (RPR) Tests for the Laboratory Diagnosis of Syphilis. Journal of clinical microbiology. 2023 Jun 20:61(6):e0016823. doi: 10.1128/jcm.00168-23. Epub 2023 May 23
[PubMed PMID: 37219422]
[278]
Tsuboi M, Nishijima T, Aoki T, Teruya K, Kikuchi Y, Gatanaga H, Oka S. Usefulness of Automated Latex Turbidimetric Rapid Plasma Reagin Test for Diagnosis and Evaluation of Treatment Response in Syphilis in Comparison with Manual Card Test: a Prospective Cohort Study. Journal of clinical microbiology. 2018 Nov:56(11):. doi: 10.1128/JCM.01003-18. Epub 2018 Oct 25
[PubMed PMID: 30135229]
[279]
Sanfilippo AM, Freeman K, Schmitz JL. Comparison of Manual and Fully Automated AIX1000 Rapid Plasma Reagin Assays for Laboratory Diagnosis of Syphilis. Journal of clinical microbiology. 2018 Aug:56(8):. doi: 10.1128/JCM.00214-18. Epub 2018 Jul 26
[PubMed PMID: 29618500]
[284]
Rockwood N, Nwokolo N. Syphilis the great pretender: when is cancer not cancer? Sexually transmitted infections. 2018 May:94(3):192-193. doi: 10.1136/sextrans-2017-053508. Epub 2018 Mar 8
[PubMed PMID: 29519910]
[285]
Zhou HY, Di Y, Ye JJ, Xu HY. [The ocular manifestations of human immunodeficiency virus and syphilis coinfection]. [Zhonghua yan ke za zhi] Chinese journal of ophthalmology. 2019 Apr 11:55(4):267-272. doi: 10.3760/cma.j.issn.0412-4081.2019.04.008. Epub
[PubMed PMID: 30982288]
[286]
Girometti N, Junejo MH, Nugent D, McOwan A, Whitlock G, 56 Dean Street Collaborative Group. Clinical and serological outcomes in patients treated with oral doxycycline for early neurosyphilis. The Journal of antimicrobial chemotherapy. 2021 Jun 18:76(7):1916-1919. doi: 10.1093/jac/dkab100. Epub
[PubMed PMID: 33783506]
[287]
Ghanem KG. Management of Adult Syphilis: Key Questions to Inform the 2015 Centers for Disease Control and Prevention Sexually Transmitted Diseases Treatment Guidelines. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2015 Dec 15:61 Suppl 8():S818-36. doi: 10.1093/cid/civ714. Epub
[PubMed PMID: 26602620]