Continuing Education Activity
This activity educates the healthcare professionals on the etiology, epidemiology, evaluation, and treatment of Ventral Cord Syndrome. The goal of this activity is to provide health-care professionals with concise and updated information on Ventral Cord Syndrome that can be translated easily into clinical practice.
Objectives:
- Identify the etiologies and pathophysiology of Ventral Cord Syndrome.
- Explain the importance of monitoring for disease complication in patients afflicted with ventral cord syndrome.
- Outline the treatment and management options available for Ventral Cord Syndrome.
- Summarize the importance of improving care coordination among the interprofessional team to enhance the delivery of care for patients with ventral cord syndrome.
Introduction
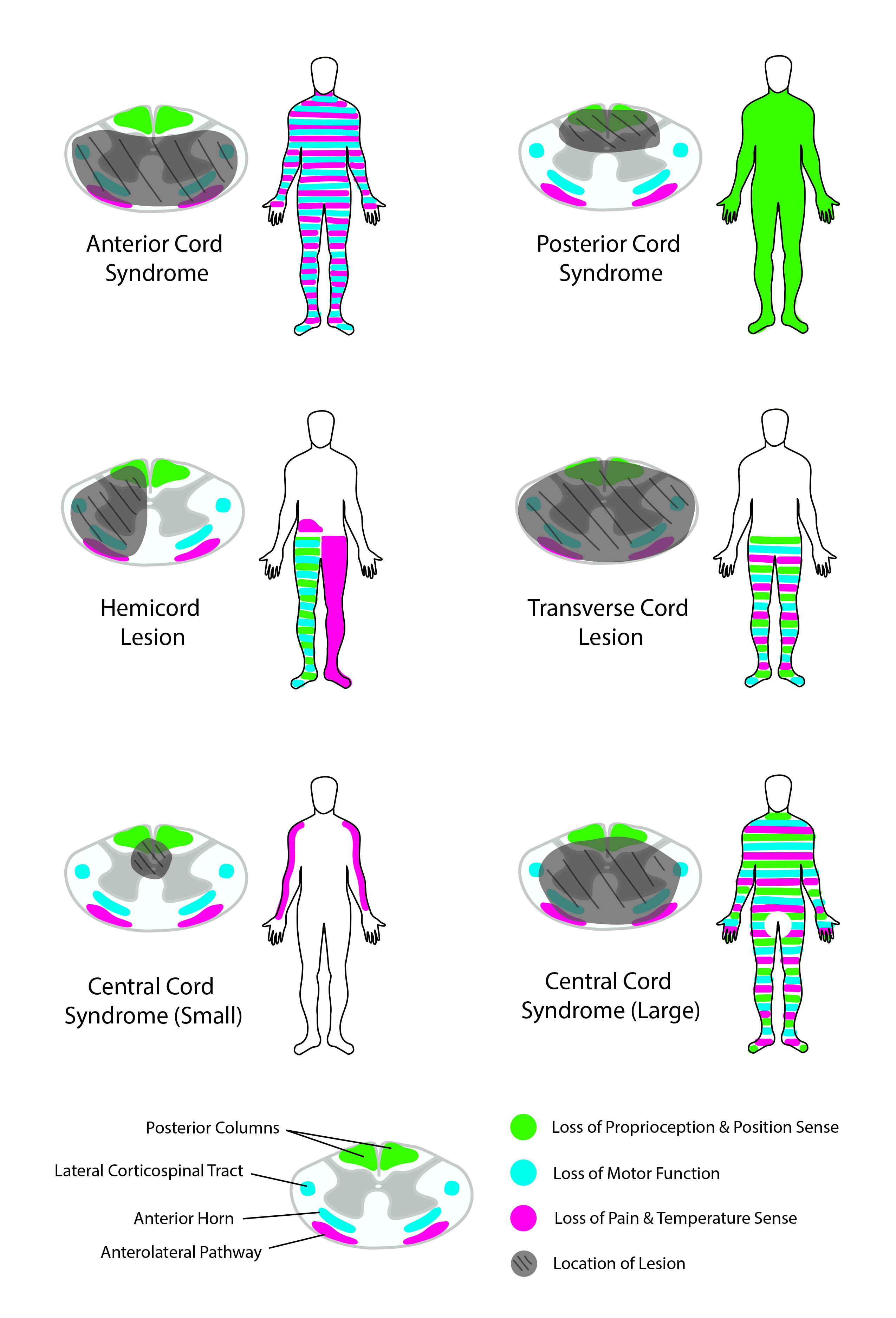
Ventral cord syndrome (VCS), also referred to as anterior cord syndrome or anterior spinal artery syndrome, is caused by any condition that leads to infarction of the ventral two-thirds of the spinal cord. Estimates for the incidence and prevalence of ventral cord syndrome vary, yet it is the most common type of spinal cord infarction. Patients with ventral cord syndrome present with impairments of both pain and temperature sensation while maintaining vibration and proprioception sensation. Motor deficits are noticeable both at and below the level of injury. Magnetic resonance imaging can provide a visual assessment of the degree of spinal cord ischemia. Current treatment for ventral cord syndrome is primarily supportive and follows guidelines for cerebral ischemia, atherosclerotic vascular disease, and acute traumatic spinal cord injury.
Etiology
Ventral cord syndrome results from direct or indirect damage to the ventral spinal cord. A direct injury arises when the spinal cord is mechanically crushed or compressed, as in the case of an enlarging hematoma. Indirect injuries constitute the primary etiology of ventral cord syndrome, and the resultant tissue damage is secondary to the ischemia. The integrity of the aorta is critical for the proper perfusion of the ventral aspect of the spinal cord and knowledge of vascular anatomy is required to understand this concept. The aorta gives rise to subclavian arteries, which in turn give rise to the vertebral arteries. The vertebral arteries give rise to the anterior spinal artery (ASA), which supplies the anterior two-thirds of the spinal cord. Therefore, any condition that compromises the aorta, such as aortic dissection, may cause indirect injuries to the ventral cord. Ventral cord syndrome can also result from iatrogenic damage to the aorta following surgical complications during attempted vascular repairs or with prolonged clamping of the aorta. Other less common causes of infarcts to the ventral cord are intrinsic arterial occlusion due to emboli or atheroma of the ASA or the radicular branches.[1]
The anterior aspect of the spinal cord is particularly susceptible to ischemia because the supplying vessels are end-arteries and typically have no anastomoses. ASA infarction may also occur due to impairment of perfusion via the great anterior radicular artery of Adamkiewicz, which is the major contributor to the caudal two-thirds of ASA in the lower thoracic and upper lumbar region (T5 to L2) of the spinal cord. Other causes of disruption of blood flow in ASA are aneurysms, trauma or syphilitic arteries.[2] Finally, hypotensive states, venous infarctions, multiple sclerosis, and cord impingement by fracture fragments are less common causes of vascular damage to the anterior cord.
Epidemiology
Currently, there are few epidemiological studies on non-traumatic spinal cord injury, and these do not allow for an accurate estimate of the actual incidence and prevalence.[3] As mentioned earlier, the leading cause of ventral cord syndrome is a non-traumatic ischemic injury. Acute spinal cord infarction is a rare disorder that accounts for only 8% of all myelopathies and not more than 1% of all strokes.[4] Ventral cord syndrome is the most common of all ischemic spinal cord syndromes. Many of the case series literature to date on the subject of ischemic lesions have relatively small cohorts; they demonstrate that the age range typically affected spans from the first to tenth decades of life with a median age in the sixth to seventh decades.[5]
Pathophysiology
The anterior two-thirds of the cord contains important tracts for the proper functioning of the central nervous system (CNS); injury impairs the actions of these tracts. Motor function impairment arises from damage to the efferent corticospinal tract and sensory deficits arise from damage to the afferent spinothalamic and spinocerebellar tracts. Other manifestations of the syndrome will depend on the location where the cord was injured. In general, there is a risk for autonomic dysreflexia, sexual impairments, movement impediments, neuropathic pain, and neurogenic bladder and bowel.[6]
The clinical onset of ventral cord syndrome is abrupt, with pain, flaccid paraplegia or tetraplegia below the lesion, and alterations in temperature and pain sensation.[7] Vibration, fine touch, and proprioception sensory modalities will not be affected, as these are relayed by the dorsal columns which are located in the posterior one-third of the cord and are supplied by the two posterior spinal arteries.
Histopathology
Ischemic and reperfusion injuries cause damage to the spinal cord by the activation of microglia and astrocytes, blood-spinal cord barrier (BSCB) disruption, tissue edema, and neutrophil influx.[8] Upon disruption of blood flow to the CNS, a complex signaling cascade triggers leading to neuronal destruction. Blood flow reduction and the resulting oxygen deprivation produces ionic pump failure and anoxic depolarization, leading to excess glutamate and intracellular calcium. Overactivation of the N-methyl-D-aspartate (NMDA) and a-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, dysregulation of metabolic and mitochondrial activity, and loss of osmotic balance ultimately result in excitotoxic cell death. This rise in calcium triggers free radical production, cytotoxic edema, and increased nitric oxide (NO) generation, which contribute to cellular damage and loss. During the subacute stage, additional neuronal death is believed to result from the accompanying inflammatory response, oxidative stress, and activation of apoptosis pathways, which, together, are also termed ‘reperfusion injury.’[9]
On the other hand, mechanical trauma to the spinal cord results in injury during two separate phases. The initial direct trauma causes acute compression and disruption of axons and vasculature. In the second phase, a cascade of events of histology gets triggered as a result of the injury, including hemorrhage, edema, inflammation, demyelination, pathologic changes in the neurons and oligodendroglia, as well as microglial and astrocyte activation in an early stage. A later stage consists of scar formation, Wallerian degeneration, development of cysts and syrinx, and schwannosis.[8]
History and Physical
Obtaining a complete history is vital in determining the etiology of ventral cord syndrome. Neurological examination shows complete motor deficiency along with the loss of pain, temperature, and crude-touch sensations. Bilateral motor deficits will be relevant directly below the level of the lesion whereas bilateral sensory deficits start two to three dermatomal segments below the level of the lesion since the spinothalamic tract ascends at least two to three segments before decussating at the anterior commissure. Early motor deficits due to spinal shock include flaccidity with absent reflexes, followed by a gradual return of the reflexes and increased tone or spasticity. Ventral cord syndrome patients will have preserved sensations of light touch, proprioception, and vibration. Damage to the autonomic centers of the spine results in orthostatic hypotension, bladder and bowel incontinence, and sexual dysfunction. Also, patients often report a radicular or diffuse type of pain.[10]
Evaluation
Magnetic resonance (MR) imaging is the imaging modality of choice for visual evaluation of spinal cord ischemia, which should include axial and sagittal diffusion-weighted MR imaging examinations. Characteristic MR imaging features of acute spinal cord ischemia include diffusion restriction in the ASA territory on diffusion-weighted images and a pencil-like hyperintense signal on sagittal T2-weighted images, with or without cord enlargement. Axial MR images show a central T2-hyperintense signal on either side of the median fissure because there is relative sparing of the peripheral and posterior cords due to collateral vessels from the vascular pial plexus and both posterior spinal arteries. In some cases, a central T2-hyperintense signal resembling "snake eyes" appears in the anterior spinal cord on either side of the median fissure. Although this finding is considered specific for spinal cord infarction, it can also appear in association with compression myelopathy and various infectious or inflammatory spinal conditions.[10] MR angiography or CT angiography are appropriate adjunctive modalities for further delineation of vascular pathology. Digital subtraction angiography will delineate the vascular anatomy, and this is necessary in cases that will require an interventional procedure.[11]
Laboratory workup consists of complete blood cell count; erythrocyte sedimentation rate; electrolyte, glucose, creatinine, and cholesterol levels; liver function test; protein electrophoresis; coagulation tests; electrocardiogram. These tests are done to screen for conditions that cause ischemic damage to vessels such as diabetes mellitus, atherosclerosis, and hypercholesterolemia. Serological tests for borreliosis, Lyme, syphilis, human immunodeficiency virus, human T-cell lymphotropic virus, and herpes virus are necessary if infections are a consideration as a cause of infarct. Patients can also have screening for antinuclear and antineutrophil cytoplasmic antibodies, which can clue into a vasculitis as a cause. The cerebrospinal fluid should undergo analysis for cell count; glucose and protein levels, and oligoclonal bands as well as for the presence of antibodies to particular pathogens. CSF analysis is likely to show increased protein concentration without pleocytosis or intrathecal synthesis of immunoglobulins.[12]
Treatment / Management
Current treatment is mainly supportive and follows guidelines for cerebral ischemia, atherosclerotic vascular disease, and acute traumatic spinal cord injury. The primary focus is to address the underlying cause, which includes fever and glycemic control, management of anticoagulation, antiplatelet and thromboprophylaxis therapy.[9]. It is paramount to protect airways and maintain blood pressure support in an acute setting. If the patient is hypotensive, then blood pressure must be raised by using phenylephrine, norepinephrine and high-dose dopamine. For blood pressure reduction, preferred medications include labetalol, esmolol, and nicardipine.[13]
Risk factors associated with more severe spinal cord strokes include hypertension, diabetes mellitus and elevated blood glucose on admission (regardless of whether the patient has diabetes mellitus). Cardiovascular risk factors are well-established etiologies of cerebral stroke. Treatment and prevention of these risk factors should be essential in acute spinal cord stroke management.[14]
Management in most cases is primarily limited to improvement of symptoms and prevention of future complications. The patient needs to receive education on how to adapt to their new body and condition. Impairments during hospitalization and after discharge must be addressed, including limitations in mobility and activities of daily living, bladder, bowel and sexual dysfunction. Pressure ulcers, pain, and spasticity are common complications that require appropriate management. After discharge, patients must obtain and be able to safely use the necessary adaptive equipment so that they can optimize their functional independence. A particular focus must be given to the patient's psychosocial function and participation to achieve increased independence, quality of life, and ultimately a better prognosis.
Studies have shown the benefit of lumbar CSF drainage along with the elevation of mean arterial pressure from 60 to 90 or 100 mmHg in avoiding ischemic injury during vascular repair surgery. Surgical maneuvers such as aortic cross-clamping and retraction can produce acute elevations in CSF pressure, which hinders venous outflow leading to ischemia. Hence, this ischemia may be reversible in some cases by utilizing CSF drainage while supporting systemic blood pressure.[15][16] Steroids can also be added to this regime to reduce both cytotoxicity and vasogenic edema. For open procedures, left heart bypass maintains perfusion to the distal aortic and iliac arteries.[9]
Differential Diagnosis
Ischemic spinal cord injury is a rare and often misdiagnosed disease. Multiple sclerosis can show similar MRI findings to spinal cord infarctions, thus risking confusion. Spinal cord neoplasms should be in the differential diagnosis though tumors typically have a slow clinical onset, unlike the acute presentation of spinal cord ischemia. Transverse myelitis is another condition with a similar clinical presentation to infarctions, yet the onset is not quite as acute. Venous congestive myelopathy should also be on the differential, yet it differs from infarcts in that it will present with prominent enlarged pial veins with the involvement of the central and peripheral white matter of the cord.[17]
Pertinent Studies and Ongoing Trials
Current preclinical research is studying promising pharmacological agents as a possible treatment for ischemic spinal cord injuries. Neuroprotective therapies have as their basis the premise of limiting secondary injury after the initial damage by modulating inflammation, reducing cell death, and guarding against excitotoxicity. There are currently no proven pharmacological therapies for human spinal cord injury that provide neuroprotection. Studies use propofol, thymoquinone, minocycline, erythropoietin, and curcumin as possible neuroprotective pharmacologic agents. Antioxidants are used to protect against the oxidative damage caused by secondary neurodegeneration after acute SCI. Antioxidants seem to stabilize mitochondrial bioenergetics, protect neurons and achieve modest neurological recover after injury.
Emerging research is focusing on measures which could help prevent ischemic spinal cord injury during vascular surgery. Hypothermia preserves tissue integrity during periods of interrupted blood flow; animal studies suggest that hypothermia suppresses excitatory synaptic transmission and reduces injury to neurons. Hyperbaric oxygen increases tissue oxygenation and metabolism in penumbral tissue. Ischemic preconditioning is a way to prepare tissue for the ischemic state, thus rendering it more resistant to a hypoxic insult. Numerous preclinical investigations have looked at the molecular pathways and potential benefits of both pre and post-ischemic preconditioning with encouraging results. Other studies are examining how to counteract apoptosis which leads to neuronal cell death.
Many of the current treatments for spinal cord infarcts show only modest improvements in the functional outcome if used as monotherapy, which is probably attributable to the fact that many factors lead to the development of permanent neurological deficits. Thus, future treatment trials should consider a combined and multimodal treatment approach.[9]
Staging
Acute spinal cord injury occurs in two phases. The initial injury phase results from the initial insult to the cord; be it by direct mechanical damage or by disruption of blood vessels, axons or cells membranes. This initial insult triggers a pathophysiological cascade that takes place within seconds. The secondary injury phase results from the on-going endogenous triggers that cause tissue destruction. The secondary phase can further subdivide into the immediate (less than 2 hrs), early acute (less than 48 hrs), and subacute (less than 2 weeks) phases.[18]
Prognosis
Spinal cord infarct patients have a better prognosis than those with cerebral stroke. A study of 30 patients with spinal cord injury looked at the long-term prognosis by following them for a mean of 7.1 years. The study concluded that long-term mortality was lower in spinal cord infarct patients after adjusting for age and functional scores in the acute phase. The better prognosis is likely because spinal cord infarction patients have lower frequencies of atrial fibrillation, hypertension, and cardiac disease.[19]The same study found that many patients with spinal cord infarction made significant clinical improvements which improved prognosis. The determination was that in the long run, most of the surviving patients over 60 years of age at the time of injury had returned to their jobs. On the other hand, re-employment was lower among stroke patients less than 60 years at the onset even when their functional scores are better than those of spinal cord infarction patients. Cerebral infarction patients are likely to have cognitive deficits, yet the prevalence of cognitive deficits is rare among spinal cord infarction patients and probably do not differ from those in other hospitalized patients with an equally serious condition.[19][14]Chronic pain is a common finding in spinal cord infarction patients with up to 79% of patients reporting chronic pain on follow-up. There is also a low incidence of depression in these patients, which is likely attributable to their preserved cognitive functioning. Higher mortality in the spinal cord infraction population is associated with older age, the severity of impairments in the acute phase and the presence of peripheral vascular disease.[14]The prognosis for ventral cord syndrome will depend in large part to the initial degree of impairment upon injury. In general, studies seem to conclude that the less severe the impairment, the better the outcome.[20][21] Further, the initial severity of the impairment, especially motor deficits, is the best predictor of functional recovery.
Complications
Ventral cord syndrome shares similar complications to other spinal cord injuries and diseases. With that said, spinal cord injury increases the risk of many medical complications and morbidities most of which result from the resulting paralysis and extreme inactivity. Physical inactivity itself is responsible for insulin resistance, carbohydrate metabolism disorders, and dyslipidemia. Calcifications in the coronary arteries are more prevalent in spinal cord injury patients as compared to their able-bodied counterparts. Patients are at increased risk for fractures and osteoporosis because of immobility. There is a reduction in circulating growth hormone and testosterone. The lifetime risk of pressure ulcers is estimated to be around 50% in patients with spinal cord injury. There are also adverse changes to soft tissue body composition with increased adiposity and a reduction in skeletal muscle. Diffuse gastrointestinal tract dysmotility often brings about other problems such as gastrointestinal reflux and difficulty in evacuations. Respiratory dysfunction can arise depending on the level and completeness of the injury. Patients with spinal cord injury above the sixth thoracic vertebra have deficits in both cardiac and peripheral vascular mechanisms which are needed to maintain blood pressure; thus they are often hypotensive. Persistent and orthostatic hypotension can predispose to cognitive impairments.[22]
Postoperative and Rehabilitation Care
Since postoperative treatments for ventral cord syndrome are often ineffectual, measures to prevent perioperative cord ischemia should include avoiding anemia, systemic hypotension, and hyperextension/hyperflexion during positioning. Efforts must be made to minimize any surgical accidents resulting in vascular dissection.[23] During procedures that involve interventions of the thoracoabdominal aorta, early detection of spinal cord ischemia can be achieved by neuromonitoring with evoked potentials in the anesthetized patient and with the neurologic examination in the awake patient.[24]
Tetraplegia and paraplegia are amongst the most devastating consequences of ventral cord syndrome. These movement impediments have a significant medical, social, and financial cost to both the individual and society. Since no current treatment can reverse ventral cord syndrome, patients must receive rehabilitation focused on improving independence and quality of life all while preventing secondary complications of spinal cord injury. Patients are candidates for several forms of rehabilitation including but not limited to physical, occupational, vocational, and neuropsychological therapies.
Deterrence and Patient Education
During hospitalization, patients require instruction on adapting to life after ventral cord syndrome. Follow-up visits also provide an opportunity for support and patient education. The journey of adjustment to disability is an evolving, dynamic, general process from which the patient progresses to an optimal state of person-environment congruence. The clinical team should focus on instructing and coaching the patient through this process.[25]
Patients must receive training in developing effective coping strategies; literature supports the role of these strategies during the psychological adjustment phase. A study by Kennedy et al. showed that 45 patients who participated in a coping training program showed lower scores for depression and anxiety compared to a control group that received the standard of care.[26]
Psychological resources are inner, health-protecting and health-promoting potentials of a person, which represent a source or means to deal with challenging situations. They may include abilities, skills, knowledge, experience, talents, strengths and behavioral patterns of a person. Clinicians must help the patient strengthen their psychological resources to support a successful adjustment.[25]
The patient must obtain counsel on the importance of sports participation and exercise as a means of improving health. The literature consistently shows that exercise also improves functional performance, enhances psychological well-being and social integration. Before engaging in physical activity, an appropriate screening must be performed to determine the proper exercise prescription, identify precautions and to provide necessary modifications to physical activity.
Education also centers around other social aspects of patient life. Clinicians play a crucial role in facilitating the individual to engage in gainful employment. The medical team needs to assess and provide options and modifications for transportation. Transportation is a vital element of independence and social integration in the spinal cord injury community.
Hospital discharge provides clinicians with an excellent opportunity to educate the patient, family, friends, and caretakers. The medical team must assess the options and equipment available that facilitate the patient's life and integration to society. Discharge planning needs to consider preparing for emergencies and complications, establishing follow-up appointments and support, providing the patient with appropriate medical equipment, and home assessment.
Enhancing Healthcare Team Outcomes
Patient participation is a core component of high-quality healthcare and is associated with improvement of self-care, functioning, and satisfaction. A literature review considers patients as experts on their bodies, symptoms, and situations. As experts, patients’ experiential knowledge is now considered to be complementary to the medical professionals’ knowledge and essential for successful treatment and improving the quality of care.[27]
Participation becomes particularly crucial during spinal cord injury rehabilitation process were the health care staff is composed of a broad range of professionals including nurses, physiotherapist, occupational therapist, social workers, and physicians. Patients need to be encouraged to engage in the management plan as a team member. A level V evidence study performed in Sweden found that information that is adapted to the individual and delivered promptly is indispensable for enabling patients to actively and meaningfully participate in decisions regarding their care and rehabilitation.[28]
Recovery from spinal cord injury relies on the care received at each point along this recovery timeline. A systematic review analyzed the treatment literature on SCI to facilitate the practice of evidence-based medicine and best clinical practice. Fehlings and co-workers summarized the most notable findings in the research and offered evidence-based care recommendations. Some of their most important proposals are as follows[29]:
- Patients should be placed on a cervical collar, head immobilizers, and a spinal board before being transported to a hospital
- MRI is a strong recommendation to have a prognosis of acute spinal cord injury
- Spinal cord injury patients are at significant risk of respiratory and cardiovascular problems so management should anticipate these potential complications
- Outcomes show more improvement by management in specialized centers with access to intensive care
Treatment of acute spinal cord injury is an interprofessional effort that begins shortly after the injury, continues during the acute care setting and in some cases will go on for the remainder of the patient's life. An interprofessional team approach that includes physicians, specialists, therapists, and specialty-trained nursing will bring about the most optimal care leading to the most satisfactory clinical outcome. [Level V]