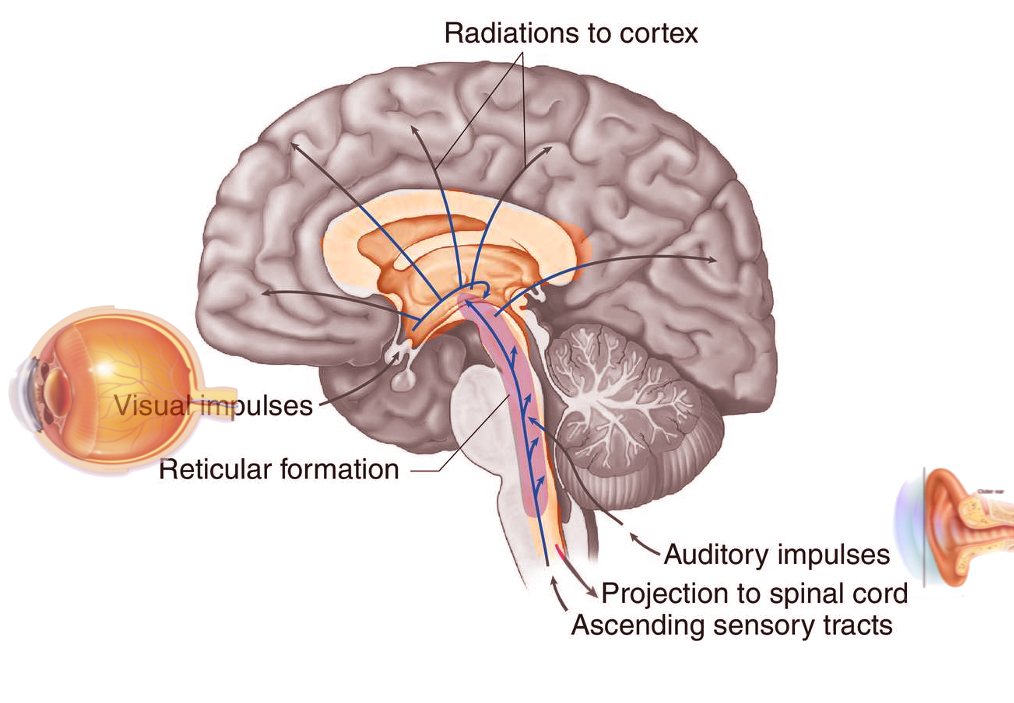
Introduction
The reticular formation is a complex network of brainstem nuclei and neurons that serve as a major integration and relay center for many vital brain systems to coordinate functions necessary for survival. The structure of the reticular formation forms a net-like connection of nuclei and neurons, hence its name “reticular,” which correlates to its function of integrating, coordinating, and influencing various regions of the central and peripheral nervous systems, both rostrally and caudally through a series of tracts. The reticular formation does not contain distinct boundaries, and the many nuclei included in this structure do not have precise delineations of territory, making the reticular formation a difficult structure to characterize and study. The widespread and diffuse nature of the reticular formation makes studies that rely on the destruction of a specific brain region difficult, and case studies of human brainstem injury do not only affect the reticular formation in isolation. These challenges inherent to the study of the reticular formation leave many questions to be answered and provide for future research opportunities.[1]
Structure and Function
The reticular formation is made up of a net-like structure of various brainstem nuclei and neurons and covers an expansive portion of the brainstem, beginning in the mesencephalon, extending caudally through the medulla oblongata, and projecting into the superior cervical spinal cord segments. The reticular formation does not have any distinct cytoarchitectural boundaries and is dispersed throughout the brainstem as a network of interconnected neurons with many projections rostrally to subcortical and cortical brain structures as well as caudally to the spinal cord. Despite having non-distinct borders, the reticular formation contains over 100 individual brainstem nuclei.[2] Within this vast array of neuronal connections, there are related but distinct brainstem nuclei, such as the red nucleus and the nucleus reticularis tegmenti pontis, embedded in the reticular network. Due to the expansive network of tracts and the interconnected structure, the reticular formation functions as an integration, relay, and coordination center for many vital life functions and controls many of the protective reflexes.[3]
Although there are no distinct borders of the reticular formation, many of its functions have been localized and correlated with general areas of the brainstem. By dividing the reticular formation into different areas based on their orientation caudally, rostrally, medially or laterally, certain areas can correlate with neuronal cell types and various functions discovered through various experiments on animal models as well as human case studies.[2] Many of the neurons in the reticular formation are multi-modal and respond to various modalities of stimuli, allowing them to integrate many different types of senses and relay them to higher cortical areas.[4] Interneurons that make up the vast majority of the neuronal population in the reticular formation allow for this vast connectivity. Each neuron within the reticular formation makes synapses with many other secondary neurons, causing an exponential number of connections to form the network-like structure.[2]
The reticular formation, through its vast array of projections and networks, functions to coordinate many reflexive and vital functions. The major functions that the reticular formation influences are arousal, consciousness, circadian rhythm, sleep-wake cycles, coordination of somatic motor movements, cardiovascular and respiratory control, pain modulation, and habituation. Cardiovascular control, in specific, is modulated by the vasomotor center present in the medulla oblongata.[2] The central areas, which research has determined to play a role in the autonomic rhythms of respiration, are located caudally in the reticular formation near the junction of the pons and the medulla. These centers are also associated with the cranial nerve motor nuclei of the trigeminal, facial, glossopharyngeal, vagus, and hypoglossal nerves to coordinate the complex task of respiration.[5]
Dividing the Reticular Formation in the Medial to Lateral Orientation
The reticular formation present in the pons and medulla can divide into lateral and medial tegmental fields, each associated with a different neuronal population and function. The lateral tegmental field of the reticular formation contains mostly populations of interneurons, which is the major cell type present throughout the entirety of the reticular formation. These interneurons in the lateral tegmental field influence many of the cranial nerve motor nuclei (trigeminal, facial, vagal, and hypoglossal), as well as form projections to various structures of the limbic system. Also, in the lateral tegmental field, premotor neurons are present that project via long descending axons to spinal cord motor neurons, which participate in many of the autonomic functions necessary for survival, such as respiration, regulation of abdominal pressure and function, micturition, and regulation of blood pressure. In contrast, the medial tegmental field of the reticular formation has the function of coordinating eye and head movements and integrating these movements with other somatosensory, vestibular, and proprioceptive stimuli through descending axonal tracts.[3]
The reticular formation can also divide into three columns based on their neuronal structure and function. These three columns from medial to lateral are the raphe nuclei, located in the midline of the reticular formation core, the gigantocellular reticular nuclei more laterally, and the parvocellular reticular nuclei, which comprises the most lateral aspect of the column system. The raphe nuclei form a central ridge of the reticular formation and play an important role in mood regulation and arousal through neurotransmission via serotonin and projections to the limbic regions. The medial column of the gigantocellular reticular nuclei is composed of larger neurons and coordinates motor movements. The most lateral of the columns comprising the parvocellular nuclei contain smaller neurons and are known to regulate respiratory function, specifically exhalation. The lateral aspects of the reticular formation also are close to various cranial nerves and work to modulate their motor function.[2]
The Ascending and Descending Tracts of the Reticular Formation
Many projections arise from the reticular formation and either ascend to subcortical and cortical regions of the brain or descend to other areas of the brainstem and spinal cord, allowing the reticular formation to play a major role as an integration and relay center. The major ascending pathway is known as the ascending reticular activating system and plays a role in establishing alertness, arousal, consciousness, sleep-wake cycles, and circadian rhythm. The ascending reticular activating system has a neuronal population consisting of mostly dopaminergic, noradrenergic, serotonergic, histaminergic, cholinergic, and glutamatergic brain nuclei, which have projections to the thalamus and cerebral cortex, primarily the prefrontal cortices. A major regulatory system of the ascending reticular activating system is the lateral hypothalamus. This region of the brain contains orexin neurons, which are key neurons in the coordination of alertness and sleep-wake cycles. Damage to this region of the brainstem results in reductions in the level of consciousness and progression to coma in many patients. If lesions affect the ascending reticular activating system bilaterally at the level of the midbrain, death can result. The ascending reticular activating system is also responsible for the phenomenon of habituation. This process allows the brain to ignore stimuli that are repetitive and meaningless and diverts focus to more important and changing stimuli in the environment.[2]
The reticulospinal tracts are the major descending pathways from the reticular formation and act on many levels of the spinal cord to coordinate movements and autonomic functions. The reticulospinal tracts project to spinal cord motor neurons and help to modulate tone, balance, posture, and coordination of body movements with the assistance of other sensory stimuli, such as visual, auditory, vestibular, and proprioceptive information. In the lateral system of the descending reticulospinal tract are the corticospinal and rubrospinal tracts, which modulate fine movement control. The medial system of the descending reticulospinal tracts is composed of the reticulospinal pathway and the vestibulospinal pathway, major players in coordination posture. This reticulospinal pathway further divides into the medial pontine and the lateral medullary reticulospinal tracts, each having a unique function. The medial pontine reticulospinal tract controls extensor musculature. The lateral medullary reticulospinal tract functions to inhibit excitatory axial extensor muscles as well as control autonomic functions of breathing.[2]
These descending pathways of the reticular formation play a major role in maintaining appropriate posture. If there is damage to the reticulospinal tract in the pons or medulla or the vestibulospinal tract, patients can experience postural instability and ataxia. Damage, which disrupts the normal signaling of the vestibular nuclei in the pons from the red nucleus located in the midbrain, may cause decerebrate posturing, causing the arms and legs to extend and internally rotate in response to painful stimuli, with hyperreflexia and hypertonic muscles. Damage to the brainstem above the red nucleus may cause decorticate posturing, in which the arms remain flexed towards the core of the body, and the legs extend in response to painful stimuli. Damage below the vestibular nuclei in the medulla may lead to hypotonia, hyporeflexia, flaccid paralysis of the limbs and body, quadriplegia, and loss of the respiratory drive. This phenomenon is called spinal shock, and patients experience these symptoms because of the loss of tonic activity from both the lateral vestibulospinal and reticulospinal tracts, which normally influence peripheral motor neurons.[2] There are also some areas of the reticular formation whose axons bifurcate and send signals in both the ascending and descending tracts. These areas are generally situated in the rostral part of the midbrain and send projections to the hypothalamus, basal ganglia, and septal areas.[1]
Dividing the Reticular Formation in the Rostral to Caudal Orientation
Another way of dividing the reticular formation into vague functional areas is in the rostral to caudal orientation. The functions of the reticular formation that are more modulatory in nature are generally controlled by the rostral sections, while the caudal sections control the premotor functions. The rostral and caudal orientation of the reticular formation also determines the relative contribution of the medial and lateral columns. As one examines the reticular formation columns moving from a rostral section more caudally, the medial reticular formation column becomes less prominent, and the lateral column becomes more prominent. Animal studies that examined the impact of lesions on various areas of the reticular formation demonstrated that rostral lesions produced hypersomnia and caudal lesions produced insomnia in cat models. Many studies such as these have taken place showing contradictory behaviors in the various regulatory functions of the reticular formation based on the location of the lesions, demonstrating its prominent role in modulation, integration, and coordinating various systems throughout the body.[2]
Pain Modulation
Another important function of the reticular formation is in the modulation of pain stimuli. For pain from the periphery to reach the cerebral cortex to be brought to conscious attention, pain signals travel through the reticular activating system through an ascending tract. The reticular activating system also projects descending pathways that play a role in the analgesic pain pathway, modulating the sensation of pain in the periphery and blocking transmission from the spinal cord to the cortex. The analgesic pain pathway works through the gate-control mechanism present in the spinal cord, in which presynaptic inhibition of pain stimulation occurs in zone II of the substantia gelatinosa of the spinal cord before it can be transmitted to a secondary neuron and ascend to the cerebral cortex via the spinothalamic tract.[2] The thought is that nociceptive stimuli reaching the reticular formation are responsible for the many behavioral and defensive responses to pain. Evidence also suggests that these ascending pain signals reaching the reticular formation in the medulla also play a modulatory role in autonomic function with a major impact on cardiovascular control as well as motor control as part of the flight or fight sympathetic reaction.[6]
Understanding the pain and analgesic pathways that are modulated by various regions of the cerebral cortex, brainstem, and spinal cord can provide crucial insights into the phenomenon of neuropathic pain. The thought is that since the reticular formation and other pain modulating regions of the brain have extensive connections to the limbic and memory centers, chronic central pain can persist despite the cessation of the noxious peripheral stimulus. Another important phenomenon relates to the reticular formations' contribution to pain following spinal cord injuries. Due to the diffuse location and multi-synaptic network of the reticular formation, it rarely is completely destroyed after a spinal cord injury, allowing for pain pathways to the cerebral cortex to persist and contributing to substantial pain and discomfort. This condition can also lead to the misinterpretation of non-painful sensations below the level of the spinal cord injury to travel through the pain conducting ascending pathways of the reticular formation, resulting in the phenomenon of allodynia.[2][6]
Ocular Responses
The reticular formation also plays a vital role in eye gaze, coordination of eye saccades, and head movement. Different parts of the reticular formation are responsible for various ocular functions. The mesencephalic reticular formation coordinates vertical gaze, the paramedian pontine reticular formation coordinates horizontal gaze, and the medullary pontine reticular formation coordinates head movements and gaze holding. These regions directly project to the extraocular motor nuclei and are essential for saccadic eye movements. These centers also have connections via the descending reticulospinal neurons to coordinate posture and neck movements with the eye movements.[3][7]
Embryology
The development of the central nervous system, which comprises the brain, brainstem, and spinal cord, initiates at gestational week three and continues after birth until late adolescence and potentially throughout the entire lifespan. After gastrulation occurs, the process in which the embryo forms a three-layered structure, the embryo begins to undergo the process of neurulation, which initiates the formation of the nervous system. The first structure created during neurulation is the neural plate, a thickened area of ectodermal tissue, which will go on to form the neural tube at approximately embryonic day 20 to 27. During the formation and growth of the neural tube, both the rostral and caudal ends of the tube close between embryonic days 25 to 27 to form a closed-off system that will develop into the structures of the central nervous system.[8]
Just prior to neural tube closure, the neural tube begins to grow outward at various regions to form three distinct pouches or the primary brain vesicles. From rostral to caudal, the three primary brain vesicles are the prosencephalon, the mesencephalon, and the rhombencephalon. After the formation of the primary brain vesicles, there are further subdivisions into the secondary five brain vesicles that go on to form the distinct structures of the mature central nervous system. The prosencephalon, the precursor to the forebrain, subdivides into the telencephalon and the diencephalon. The mesencephalon does not undergo a further subdivision and gives rise to the midbrain. The rhombencephalon subdivides into the metencephalon, which goes on to form the pons and cerebellum, and the myelencephalon, which forms the medulla. The reticular formation extends from the medulla up to the region of the brainstem caudal to the diencephalon, thus arising from regions of the mesencephalon, metencephalon, and myelencephalon.[8]
Blood Supply and Lymphatics
There is a profuse blood supply to the reticular formation due to the diffuse and expansive location of the network of brainstem nuclei and neurons. Blood supply to the brainstem, and thus, to the reticular formation, originates from the vertebrobasilar system or posterior circulation of the brain. The beginning of the posterior brain circulation system arises from the vertebral arteries, branches of the subclavian arteries. The vertebral arteries take a superior course towards the brain through the transverse foramen of the cervical vertebra. As the vertebral arteries then travel into the dural space, in an anteromedial direction through the foramen magnum of the skull, they unite to form a single basilar artery. Before the union of the vertebral arteries, the posterior inferior cerebellar artery, as well as the anterior spinal artery, arise, supplying blood flow to the inferior cerebellar hemispheres bilaterally, the cerebellar vermis and tonsils, the lateral medulla oblongata, and the upper cervical spinal cord.
After the union of the vertebral arteries, the basilar artery continues a superior course in front of the medulla and pons. It will later bifurcate at the junction of the pons and mesencephalon to give rise to the posterior cerebral arteries, supplying the occipital cortex as part of the posterior brain circulatory system. Prior to the division into the posterior cerebral arteries, the basilar artery gives off the anterior inferior cerebellar arteries, the pontine perforating branches, and the superior cerebellar arteries. The basilar artery and its subsequent branches supply the rest of the brainstem, including the medulla and pons, the midbrain, and superior structures such as the thalamus, posterior internal capsule, the middle and upper cerebellum as well as the cerebellar vermis.[9]
Clinical Significance
Hypersomnia and Coma
The reticular activating system is one of the major contributors to arousal and consciousness. A devastating consequence of increased intracranial pressure from numerous etiologies is the herniation of brain tissue inferiorly to compress brainstem structures. The degree of herniation determines the severity of impact on the arousal function of the reticular activating system, ranging from hypersomnolence to coma and potentially death. Hypersomnia is a common outcome post-stroke, and presentation correlates with stroke patients who have infarcts in the thalamus, hypothalamus, and pons. Other disturbances of the ascending reticular activating system, such as patients with narcolepsy post mild TBI and spontaneous subarachnoid hemorrhage, also showed hypersomnia as one of the associated symptoms.[10]
Schizophrenia
Many symptoms of schizophrenia, particularly the positive symptoms, such as hallucinations, sensory gating abnormalities, and sleep-wake disturbances, which are hallmarks of the disease, are thought to be due to reticular activating system abnormalities. The sleep-wake disturbances that many patients with schizophrenia experience are reductions of slow-wave sleep duration and a decrease in REM latency. There is also speculation that hallucinations may be REM intrusion into a waking state, causing these visual and auditory phenomena. Post-mortem analyses found that patients with schizophrenia had a greater than 60% increase in the number of neurons in the pedunculopontine nucleus, part of the reticular formation in the posterior midbrain, compared to patients without the disorder. The pedunculopontine nucleus typically sends excitatory projections to the substantia nigra, which in turn sends dopaminergic projections to various regions of the striatum. The significant increase in cholinergic neurons in the pedunculopontine nucleus may explain the sleep-wake disturbances since the cholinergic output component of the reticular activating system would be increased. The increase in cholinergic neurons in the pedunculopontine nucleus could also lead to an increase in the excitation of the substantia nigra, leading to excess dopaminergic output from that system. The thought is that this increase in neuronal number in the pedunculopontine nucleus is due to the failure of appropriately programmed cell death during nervous system development.[11][12]
Post-Traumatic Stress Disorder
Individuals with Post-Traumatic Stress Disorder (PTSD) often experience many symptoms related to hyperarousal, flashbacks, anxiety, and disturbances in sleep-wake cycles. Patients with PTSD experience an exaggerated startle response, often to non-harmful auditory stimuli, a phenomenon called sensory gating abnormalities. These patients also have increased REM drive and display many flashbacks and re-experiencing episodes, potentially progressing to hallucinations of past events. A study, including combat veterans with diagnosed PTSD, showed a decreased habituation of the P1 auditory evoked potential, and the degree of decreased habituation was associated with the severity of the patients’ flashback symptoms. Individuals who suffered from anxiety disorders comorbid with a diagnosis of PTSD showed a decrease of 50% of the neurons in the locus coeruleus, a part of the reticular activating system that responds to stress and panic with neurotransmission of norepinephrine. The locus coeruleus normally inhibits the pedunculopontine nucleus. Since patients with anxiety disorders and PTSD showed a significant reduction in neurons in the locus coeruleus, there may be disinhibition of the pedunculopontine nucleus, resulting in many of the symptoms of PTSD.[11]
Parkinson’s Disease
Parkinson’s disease, a progressive neurodegenerative disorder that affects the dopaminergic neurons principally in the substantia nigra, leads to many disruptions in normal movement. These difficulties with movement are displayed by a resting tremor, typically a “pill-rolling” movement of the fingers, progressive stiffness, bradykinesia, cogwheel rigidity, and difficulty initiating or changing movements. In addition to the degeneration of the substantia nigra, individuals with Parkinson’s Disease also have locus coeruleus cell death. Similar to the findings in patients with PTSD, individuals with Parkinson disease display decreased habituation of the P1 auditory evoked potential, a finding which correlates with the severity of their Parkinsonian symptoms. Research also found that the pedunculopontine nucleus is overactive in late-stage Parkinson disease. As the disease progresses, patients typically begin showing decreased habituation of reflexes and symptoms of anxiety and depression, which is a pattern similar to PTSD and schizophrenia, as described above. Patients with Parkinson disease also report having sleep-wake disturbances and REM sleep behavior disorders common in patients with Parkinson disease. The loss of locus coeruleus neurons and the subsequent disinhibition of the pedunculopontine nucleus may explain many arousal and motor symptoms present in Parkinson disease.[11] Individuals who had both poor postural control and sleep disruptions demonstrated longer duration of anticipatory postural adjustments during the initiation of gait and decreased functional activity between the pedunculopontine nucleus and the supplementary motor area in the locomotor network.[13] In another study of REM behavior disorder and Parkinson disease, smaller volumes of the pontomesencephalic tegmentum, medullary reticular formation, hypothalamus, thalamus, putamen, amygdala, and anterior cingulate cortex were noted in patients with Parkinson disease. This reduction in volume coupled with animal studies that demonstrated that lesions in the medullary reticular formation are associated with loss of REM muscle paralysis and the development of REM Behavior Disorder points to dysfunction of the reticular activating system as being an integral component in the development of this disorder.[14] By understanding how the reticular formation plays a role in the pathogenesis of both motor and sleep-wake dysfunction in Parkinson disease, the greater the number of treatment targets can be researched and implemented. Currently, experimental deep brain stimulation devices targeting the pedunculopontine nucleus are in research for improving axial motor deficits such as gait freezing and falling that is associated with the progression of Parkinson disease.[15]
REM Behavior Disorder
REM Behavior Disorder is a sleep disorder characterized by the patient acting out vivid, unpleasant, and often violent dreams. Normally during REM sleep, individuals cannot enact dreams due to muscle paralysis. In patients with REM Behavior Disorder, research showed a greater than 75% reduction in the number of neurons in the locus coeruleus. This reduction in neurons leads to a disinhibited pedunculopontine nucleus, which may offer insight as to why these patients can act out dreams. Approximately 40% of patients with REM behavior disorder receive a diagnosis with Parkinson disease ten years after the initial diagnosis of REM behavior disorder. [11]
Narcolepsy
Disorders of the reticular activating system have implications in the pathophysiology of narcolepsy. The hallmarks of narcolepsy are the presence of excessive daytime sleepiness, fragmented sleep, and cataplexy, which is the phenomenon of frequent sleep attacks. Patients also often describe having sleep paralysis, hypnagogic or hypnopompic hallucinations, and nocturnal dyssomnia. The thinking is that the dysregulation of hypocretin/orexin signaling contributes to a large portion of cases of narcolepsy.[16] In one study, patients with narcolepsy had significantly decreased, or completely absent P1 auditory evoked potential. Symptoms of narcolepsy may be due to the decreased output of the reticular activating system, potentially the pedunculopontine nucleus in specific.[11] Another study demonstrated that lesions in the pedunculopontine/laterodorsal tegmental nuclei cause decreased output and loss of specific orexin peptides, potentially explaining the excessive daytime sleepiness that patients with narcolepsy experience.[2] There have also been studies using quantitative MRI comparing brainstem structures of patients with narcolepsy to healthy participants. These studies found that there is a significantly lower R2 relaxation rate in the rostral reticular formation near the superior cerebellar peduncle in patients with narcolepsy. R2 relaxation rates are sensitive for metal ion chelating elements, such as neuromelanin. Given the location of these findings as well as the connections made with orexin projections from the hypothalamus, researchers speculate that the area of dysfunction could be the locus coeruleus or another neuromelanin-containing nucleus. This abnormality of the reticular activating system may explain the mechanisms of sleep disturbances in narcolepsy.[16]
Spinal Cord Injury
Individuals who suffer from transverse injury to the spinal cord have dysfunction related to the longitudinal tracts relaying information to and from the cerebral cortices. Spinal cord injuries have the potential of damaging the reticular formation if they localize to the brainstem. If they do not directly injure the reticular formation, they disrupt the connection and thus the modulation between the reticular formation and the spinal motor neurons. The traditional signs of upper motor neuron injury, such as hyperreflexia and hypertonia, can be explained by this dysregulation between the reticular formation and motor neurons. Due to the lack of effective communication between the reticular formation and the spinal cord, the muscles below the spinal cord injury reflexively contract in response to peripheral stimuli due to spinal reflexes. Since there is no modulation and inhibition of contraction by the reticular formation, muscles inappropriately experience increased tone and spasticity. Individuals who have a spinal cord injury also have difficulties with autonomic functions, such as neurogenic bladder and dysfunction of the cardiorespiratory system.[2]
Developmental Influences
Both preterm birth and prenatal exposure to cigarette smoking have correlations with abnormalities of the reticular formation. Individuals who are born preterm demonstrate disruptions of the pre-attentional, attentional, and cortical projections of the reticular activating system throughout their development into childhood and adolescence. Symptoms associated with abnormalities in arousal, difficulties with sleep-wake patterns, and delays in reaction time commonly present. Fetuses exposed to cigarette smoke demonstrate lasting deficits in arousal, attention, as well as many cognitive deficits, which may be explained by the presence of nicotinic receptors on neurons in the pedunculopontine nucleus, leading to hyperpolarization-activated cation currents. These deficits may lead to lasting attentional dysfunction later in life.[2] Another association with development and the reticular activating system is present in Sudden Infant Death Syndrome (SIDS). Post-mortem analysis of patients who expired due to SIDS demonstrated an abnormally large number of dendritic spines in the medullary reticular formation. Normally, the peak number of dendritic spines in this region of the reticular formation occurs at 34 to 36 weeks of gestation, and dendritic spines rapidly decrease in number after this peak. Researchers hypothesize that this persistence of dendritic spines demonstrates incomplete development of the reticular activating system, potentially leading to dysfunction in higher levels of respiratory control, contributing to the pathophysiology of SIDS.[17]