Introduction
The Frank-Starling relationship is based on the link between the initial length of myocardial fibers and the force generated by contraction. There is a predictable relationship between the length between sarcomeres and the tension of the muscle fibers. There is an optimal length between sarcomeres at which the tension in the muscle fiber is greatest, resulting in the greatest force of contraction. If sarcomeres are closer together or further apart compared to this optimal length, there will be a decrease in contraction tension and strength.
The greater the ventricular diastolic volume, the more the myocardial fibers are stretched during diastole. Within a normal physiologic range, the more the myocardial fibers are stretched, the greater the tension in the muscle fibers and the greater force of contraction of the ventricle when stimulated. The Frank-Starling relationship is the observation that ventricular output increases as preload (end-diastolic pressure) increases.[1][2][3]
Mechanism
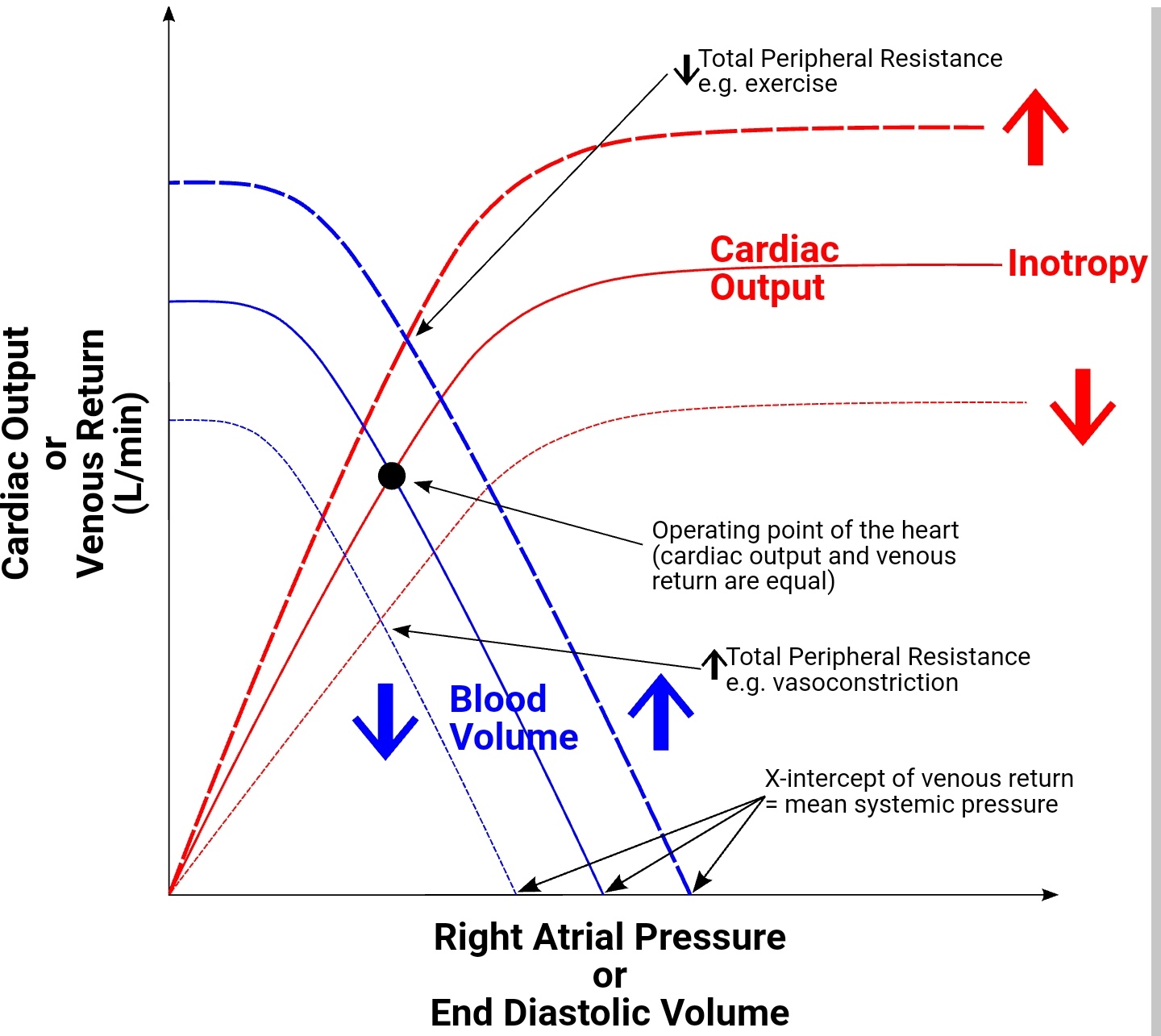
The left ventricular performance (Frank-Starling) curves relate preload, measured as left ventricular end-diastolic volume (EDV) or pressure, to cardiac performance, measured as ventricular stroke volume or cardiac output. On the curve of a normally functioning heart, cardiac performance increases continuously as preload increases. During states of increased left ventricular contractility, for example, due to norepinephrine infusion, there is a greater cardiac performance for a given preload. This is represented graphically as an upward shift of the normal curve. Conversely, during states of decreased left ventricular contractility associated with systolic heart failure, there is decreased cardiac performance for a given preload as compared to the normal curve. This is represented by a downward shift of the normal curve. Decreased contractility also can result from a loss of myocardium as with myocardial infarction, beta-blockers (acutely), non-dihydropyridine Ca++ channel blockers, and dilated cardiomyopathy.[4][5][6]
Changes in afterload, which is the force of resistance that the ventricle must overcome to empty contents at the beginning of systole, will also shift the Frank-Starling curve. A decrease in afterload will cause an upward shift of the ventricular performance curve in a similar fashion to an increase in inotropy. Conversely, an increase in afterload will cause a downward shift of the curve in a similar fashion to a decrease in inotropy.
An increase in catecholamines, such as norepinephrine, during exercise, will result in an upward shift of the Frank-Starling curve. Catecholamines achieve this increase by binding to a myocyte beta1-adrenergic receptor, a G protein-coupled receptor, ultimately resulting in increased Ca++ channel release from the sarcoplasmic reticulum, which enhances the force of contraction.[7][8]
Clinical Significance
The Frank-Starling mechanism plays a role in the compensation of systolic heart failure, buffering the fall in cardiac output to help preserve sufficient blood pressure to perfuse the vital organs. Heart failure caused by the impaired contractile function of the left ventricle causes a downward shift of the left ventricular performance curve. At any given preload, the stroke volume will be decreased as compared to normal. This reduced stroke volume leads to incomplete left ventricular emptying. Consequently, the volume of blood that accumulates in the left ventricle during diastole is greater than normal. The amplified residual volume increases the stretch of the myocardial fibers and induces a greater stroke volume with the next contraction via the Frank-Starling mechanism. This allows for better emptying of the enlarged left ventricle and preserves cardiac output.[9]
The benefit of the Frank-Starling mechanism in the compensation of systolic heart failure is limited. In severe heart failure with a greater cardiac contractility malfunction, the ventricular performance curve may be nearly flat at higher diastolic volumes, reducing the increased cardiac output with increases in chamber filling. In this circumstance, a severe elevation at the EDV and left ventricular EDP may result in pulmonary congestion.
The Frank-Starling mechanism also plays a compensatory role in patients with dilated cardiomyopathy. There is commonly dilation of both the right and left ventricles with decreased contractile function in dilated cardiomyopathy. As impaired myocyte contractility results in depression of ventricular stroke volume and cardiac output, the Frank-Starling mechanism has compensatory effects. As the elevated ventricular diastolic volume increases the stretch on the myocardial fibers, there will be a subsequent increase in stroke volume. Along with the Frank-Starling mechanism, neurohormonal activation mediated by the sympathetic nervous system also compensates for dilated cardiomyopathy by increasing heart rate and contractility, helping to buffer the decreased cardiac output. These compensatory mechanisms may lead to a lack of symptoms during the early stages of ventricular dysfunction. With progressive myocyte degeneration and volume overload, clinical symptoms of systolic heart failure will develop.
In patients with impaired myocardial systolic failure, clinicians use inotropic drugs to increase the force of ventricular contraction. Pharmacologic inotropic agents include cardiac glycosides, such as digitalis; sympathomimetic amines such as dopamine and epinephrine; and phosphodiesterase-3 inhibitors, such as milrinone. They all work through different mechanisms to enhance cardiac contraction by increasing the intracellular calcium concentration, enhancing actin and myosin interaction. This will have the hemodynamic effect of shifting a depressed ventricular performance (Frank-Starling) curve in an upward direction toward normal. At a given preload (left ventricular EDP), the stroke volume and cardiac output are increased.
With the progressive loss of ventricular contractility, increased preload (pressure) in the left ventricle will surpass the hydrostatic forces of the pulmonary venous system, resulting in pulmonary congestion. In a patient suffering from systolic heart failure with reduced ejection fraction and resultant pulmonary congestion, treatment with a diuretic, such as furosemide or hydrochlorothiazide, or a pure venous vasodilator, such as nitrates, reduces the preload without much change in stroke volume. This is because the Frank-Starling curve is almost horizontal at higher preload levels in a patient whose curve is shifted downward due to systolic contractile dysfunction. However, excessive diuresis or venous vasodilation can result in an unwanted fall in stroke volume, resulting in hypotension.
Arteriolar vasodilation therapy, like hydralazine, also has value when treating systolic heart failure with pulmonary congestion. Arteriolar vasodilators result in a decrease in afterload, allowing for an increase in stroke volume—the improved left ventricular emptying results in a decreased preload and improvement of pulmonary symptoms. There is the potential added benefit of combining treatment with a vasodilator and a positive inotropic agent, allowing for a larger increase in stroke volume than would be seen with monotherapy. Even with combination therapy with a vasodilator and inotropic agent, the Frank-Starling curve will not improve to the performance level of a normal ventricle.