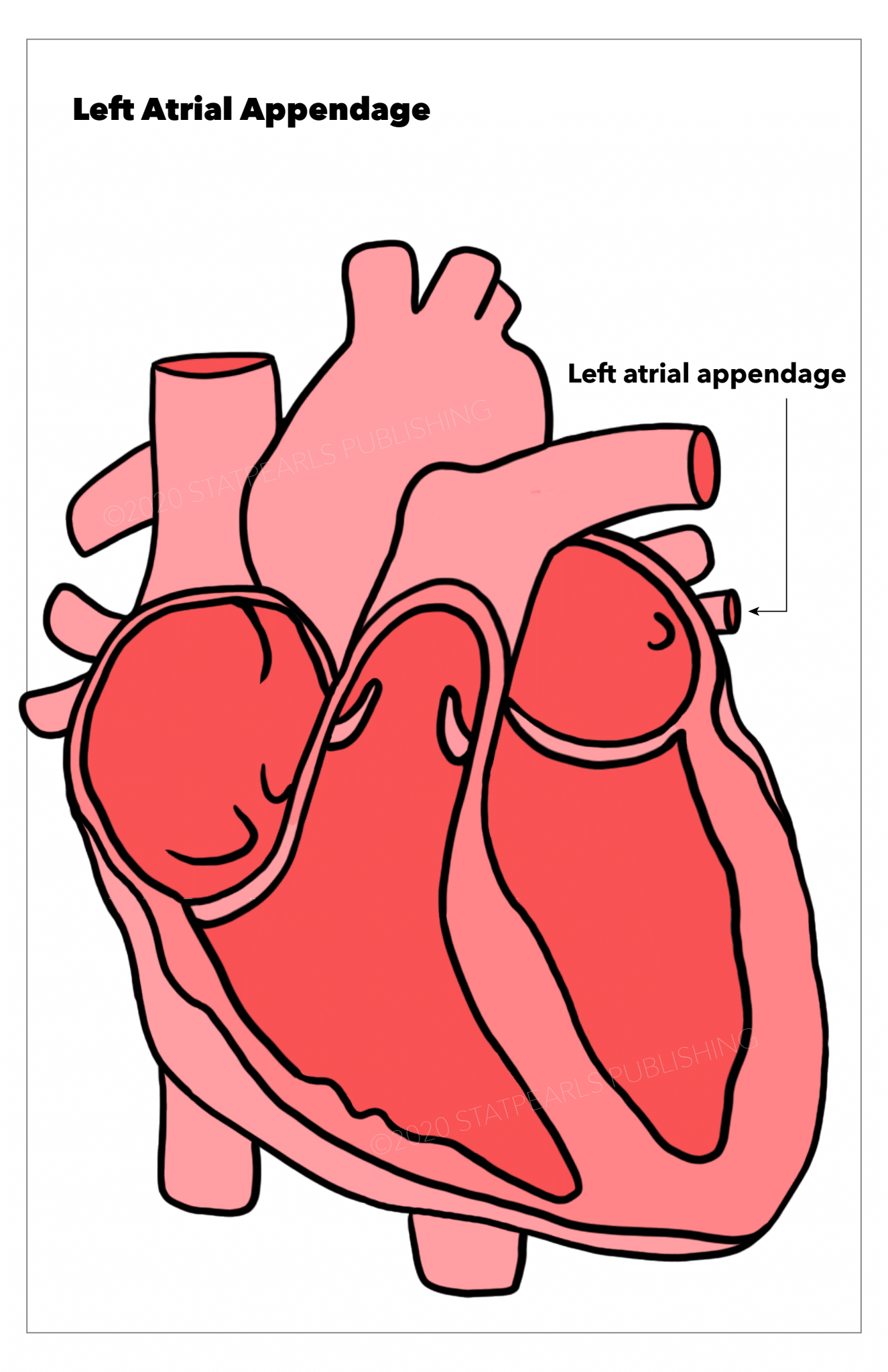
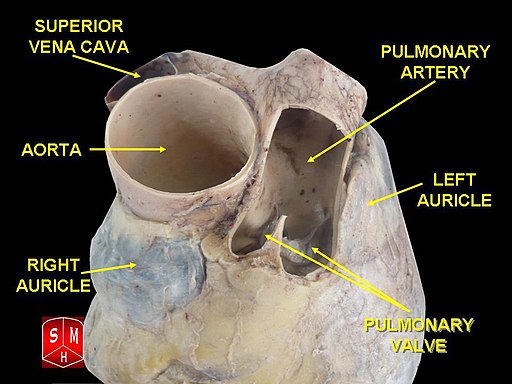
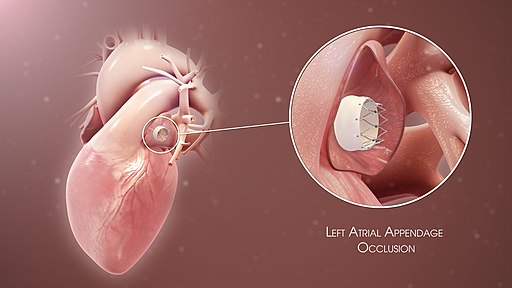
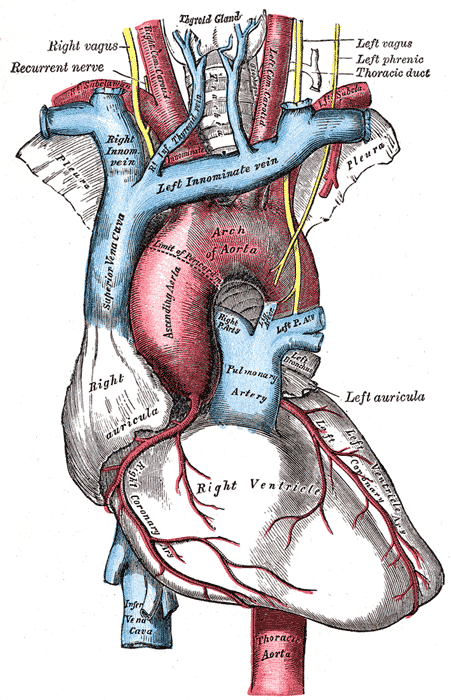
[1]
Al-Saady NM, Obel OA, Camm AJ. Left atrial appendage: structure, function, and role in thromboembolism. Heart (British Cardiac Society). 1999 Nov:82(5):547-54
[PubMed PMID: 10525506]
[2]
Di Biase L, Natale A, Romero J. Thrombogenic and Arrhythmogenic Roles of the Left Atrial Appendage in Atrial Fibrillation. Circulation. 2018 Oct 30:138(18):2036-2050. doi: 10.1161/CIRCULATIONAHA.118.034187. Epub
[PubMed PMID: 30372144]
[3]
Di Biase L, Santangeli P, Anselmino M, Mohanty P, Salvetti I, Gili S, Horton R, Sanchez JE, Bai R, Mohanty S, Pump A, Cereceda Brantes M, Gallinghouse GJ, Burkhardt JD, Cesarani F, Scaglione M, Natale A, Gaita F. Does the left atrial appendage morphology correlate with the risk of stroke in patients with atrial fibrillation? Results from a multicenter study. Journal of the American College of Cardiology. 2012 Aug 7:60(6):531-8. doi: 10.1016/j.jacc.2012.04.032. Epub
[PubMed PMID: 22858289]
Level 2 (mid-level) evidence
[4]
Wang Y, Di Biase L, Horton RP, Nguyen T, Morhanty P, Natale A. Left atrial appendage studied by computed tomography to help planning for appendage closure device placement. Journal of cardiovascular electrophysiology. 2010 Sep:21(9):973-82. doi: 10.1111/j.1540-8167.2010.01814.x. Epub
[PubMed PMID: 20550614]
[5]
Beigel R, Wunderlich NC, Ho SY, Arsanjani R, Siegel RJ. The left atrial appendage: anatomy, function, and noninvasive evaluation. JACC. Cardiovascular imaging. 2014 Dec:7(12):1251-65. doi: 10.1016/j.jcmg.2014.08.009. Epub
[PubMed PMID: 25496544]
[6]
Majunke N, Sandri M, Adams V, Daehnert I, Mangner N, Schuler G, Moebius-Winkler S. Atrial and Brain Natriuretic Peptide Secretion After Percutaneous Closure of the Left Atrial Appendage With the Watchman Device. The Journal of invasive cardiology. 2015 Oct:27(10):448-52
[PubMed PMID: 25999139]
[8]
Curry FR. Atrial natriuretic peptide: an essential physiological regulator of transvascular fluid, protein transport, and plasma volume. The Journal of clinical investigation. 2005 Jun:115(6):1458-61
[PubMed PMID: 15931381]
[9]
Potter LR, Yoder AR, Flora DR, Antos LK, Dickey DM. Natriuretic peptides: their structures, receptors, physiologic functions and therapeutic applications. Handbook of experimental pharmacology. 2009:(191):341-66. doi: 10.1007/978-3-540-68964-5_15. Epub
[PubMed PMID: 19089336]
[10]
Mukoyama M, Nakao K, Hosoda K, Suga S, Saito Y, Ogawa Y, Shirakami G, Jougasaki M, Obata K, Yasue H. Brain natriuretic peptide as a novel cardiac hormone in humans. Evidence for an exquisite dual natriuretic peptide system, atrial natriuretic peptide and brain natriuretic peptide. The Journal of clinical investigation. 1991 Apr:87(4):1402-12
[PubMed PMID: 1849149]
[11]
Sherif HM. The developing pulmonary veins and left atrium: implications for ablation strategy for atrial fibrillation. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2013 Nov:44(5):792-9. doi: 10.1093/ejcts/ezt098. Epub 2013 Feb 27
[PubMed PMID: 23447471]
[12]
Regazzoli D, Ancona F, Trevisi N, Guarracini F, Radinovic A, Oppizzi M, Agricola E, Marzi A, Sora NC, Della Bella P, Mazzone P. Left Atrial Appendage: Physiology, Pathology, and Role as a Therapeutic Target. BioMed research international. 2015:2015():205013. doi: 10.1155/2015/205013. Epub 2015 Jul 7
[PubMed PMID: 26236716]
[13]
AlTurki A, Huynh T, Dawas A, AlTurki H, Joza J, Healey JS, Essebag V. Left atrial appendage isolation in atrial fibrillation catheter ablation: A meta-analysis. Journal of arrhythmia. 2018 Oct:34(5):478-484. doi: 10.1002/joa3.12095. Epub 2018 Jul 20
[PubMed PMID: 30327692]
Level 1 (high-level) evidence
[14]
Whitlock RP, Belley-Cote EP, Paparella D, Healey JS, Brady K, Sharma M, Reents W, Budera P, Baddour AJ, Fila P, Devereaux PJ, Bogachev-Prokophiev A, Boening A, Teoh KHT, Tagarakis GI, Slaughter MS, Royse AG, McGuinness S, Alings M, Punjabi PP, Mazer CD, Folkeringa RJ, Colli A, Avezum Á, Nakamya J, Balasubramanian K, Vincent J, Voisine P, Lamy A, Yusuf S, Connolly SJ, LAAOS III Investigators. Left Atrial Appendage Occlusion during Cardiac Surgery to Prevent Stroke. The New England journal of medicine. 2021 Jun 3:384(22):2081-2091. doi: 10.1056/NEJMoa2101897. Epub 2021 May 15
[PubMed PMID: 33999547]
[15]
Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010 Feb:137(2):263-72. doi: 10.1378/chest.09-1584. Epub 2009 Sep 17
[PubMed PMID: 19762550]
Level 3 (low-level) evidence
[16]
Page RL. The Closing Argument for Surgical Left Atrial Appendage Occlusion. The New England journal of medicine. 2021 Jun 3:384(22):2154-2155. doi: 10.1056/NEJMe2106069. Epub 2021 May 15
[PubMed PMID: 33999543]
[17]
Mahmood E, Matyal R, Mahmood F, Xu X, Sharkey A, Chaudhary O, Karani S, Khabbaz K. Impact of Left Atrial Appendage Exclusion on Short-Term Outcomes in Isolated Coronary Artery Bypass Graft Surgery. Circulation. 2020 Jul 7:142(1):20-28. doi: 10.1161/CIRCULATIONAHA.119.044642. Epub 2020 Jun 3
[PubMed PMID: 32489114]
[18]
Holmes DR, Reddy VY, Turi ZG, Doshi SK, Sievert H, Buchbinder M, Mullin CM, Sick P, PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet (London, England). 2009 Aug 15:374(9689):534-42. doi: 10.1016/S0140-6736(09)61343-X. Epub
[PubMed PMID: 19683639]
Level 1 (high-level) evidence
[19]
Holmes DR Jr, Kar S, Price MJ, Whisenant B, Sievert H, Doshi SK, Huber K, Reddy VY. Prospective randomized evaluation of the Watchman Left Atrial Appendage Closure device in patients with atrial fibrillation versus long-term warfarin therapy: the PREVAIL trial. Journal of the American College of Cardiology. 2014 Jul 8:64(1):1-12. doi: 10.1016/j.jacc.2014.04.029. Epub
[PubMed PMID: 24998121]
Level 1 (high-level) evidence
[20]
López-López JA, Sterne JAC, Thom HHZ, Higgins JPT, Hingorani AD, Okoli GN, Davies PA, Bodalia PN, Bryden PA, Welton NJ, Hollingworth W, Caldwell DM, Savović J, Dias S, Salisbury C, Eaton D, Stephens-Boal A, Sofat R. Oral anticoagulants for prevention of stroke in atrial fibrillation: systematic review, network meta-analysis, and cost effectiveness analysis. BMJ (Clinical research ed.). 2017 Nov 28:359():j5058. doi: 10.1136/bmj.j5058. Epub 2017 Nov 28
[PubMed PMID: 29183961]
Level 1 (high-level) evidence
[21]
Perrotta L, Bordignon S, Dugo D, Fürnkranz A, Konstantinou A, Ricciardi G, Pieragnoli P, Schmidt B, Chun KJ. Complications From Left Atrial Appendage Exclusion Devices. Journal of atrial fibrillation. 2014 Jun-Jul:7(1):1034. doi: 10.4022/jafib.1034. Epub 2014 Jun 30
[PubMed PMID: 27957078]
[22]
Sakellaridis T, Argiriou M, Charitos C, Tsakiridis K, Zarogoulidis P, Katsikogiannis N, Kougioumtzi I, Machairiotis N, Tsiouda T, Arikas S, Mpakas A, Beleveslis T, Koletas A, Zarogoulidis K. Left atrial appendage exclusion-Where do we stand? Journal of thoracic disease. 2014 Mar:6 Suppl 1(Suppl 1):S70-7. doi: 10.3978/j.issn.2072-1439.2013.10.24. Epub
[PubMed PMID: 24672702]
[23]
Lakkireddy D, Turagam M, Afzal MR, Rajasingh J, Atkins D, Dawn B, Di Biase L, Bartus K, Kar S, Natale A, Holmes DJ Jr. Left Atrial Appendage Closure and Systemic Homeostasis: The LAA HOMEOSTASIS Study. Journal of the American College of Cardiology. 2018 Jan 16:71(2):135-144. doi: 10.1016/j.jacc.2017.10.092. Epub
[PubMed PMID: 29325636]
[24]
Yaghi S, Song C, Gray WA, Furie KL, Elkind MS, Kamel H. Left Atrial Appendage Function and Stroke Risk. Stroke. 2015 Dec:46(12):3554-9. doi: 10.1161/STROKEAHA.115.011273. Epub 2015 Oct 27
[PubMed PMID: 26508750]
[25]
Salooja MS, Singla M, Srivastava A, Mukherjee KC. Isolated tear in left atrial appendage due to blunt trauma chest: A rare case report. Journal of the Saudi Heart Association. 2013 Apr:25(2):95-7. doi: 10.1016/j.jsha.2012.11.001. Epub 2012 Nov 24
[PubMed PMID: 24174854]
Level 3 (low-level) evidence
[26]
Scorpio RJ, Wesson DE, Smith CR, Hu X, Spence LJ. Blunt cardiac injuries in children: a postmortem study. The Journal of trauma. 1996 Aug:41(2):306-9
[PubMed PMID: 8760541]