Continuing Education Activity
Interferons were discovered during investigations into virus interference and have since become essential in treating hepatitis C, cancers, and immune-mediated disorders such as multiple sclerosis. Although interferon therapy is considered effective, it has the potential to induce adverse effects on different systems within the body. A significant adverse effect associated with interferon is retinopathy, identified by flame-shaped retinal hemorrhages, cotton wool spots, and macular edema observed during funduscopic examination. Interferon-induced retinopathy is a potential complication of interferon therapy that may cause temporary and asymptomatic effects. However, due to the risk of vision loss, it is important to take a vigilant approach to prevention and early detection.
Determining whether to continue interferon treatment or opt for a dose reduction can be challenging due to the various manifestations that may arise. These manifestations may be caused by the occlusion of retinal capillaries or other ischemic etiologies due to immune complex deposition. Although less likely, severe adverse effects such as retinal artery and vein occlusion and optic neuritis are possible. This activity discusses the etiology, pathophysiology, evaluation, prevention, and management of interferon-induced retinopathy. Healthcare professionals can use this information to screen for retinopathy and make informed decisions on treatment adjustments.
Objectives:
Identify the clinical manifestations and risk factors associated with interferon-induced retinopathy by recognizing characteristic features such as retinal hemorrhages and cotton wool spots during funduscopic examination.
Screen patients undergoing interferon therapy for early signs of retinopathy through regular ophthalmologic examinations.
Implement evidence-based interventions and management strategies for individuals diagnosed with interferon-induced retinopathy.
Collaborate with ophthalmologists and other healthcare providers to communicate potential risks and benefits of interferon therapy with patients and manage interferon-induced retinopathy.
Introduction
Interferons were discovered in 1957 by Issacs and Lindenmann during investigations into virus interference and have since become essential in treating hepatitis C, cancers, and immune-mediated disorders such as multiple sclerosis. Although interferon therapy is considered effective, it has the potential to induce adverse effects on different systems within the body. A significant adverse effect associated with interferon is retinopathy, identified by flame-shaped retinal hemorrhages, cotton wool spots, and macular edema observed during funduscopic examination. Interferon-induced retinopathy is a potential complication of interferon therapy that may cause temporary and asymptomatic effects. However, due to the risk of vision loss, it is important to take a vigilant approach to prevention and early detection.
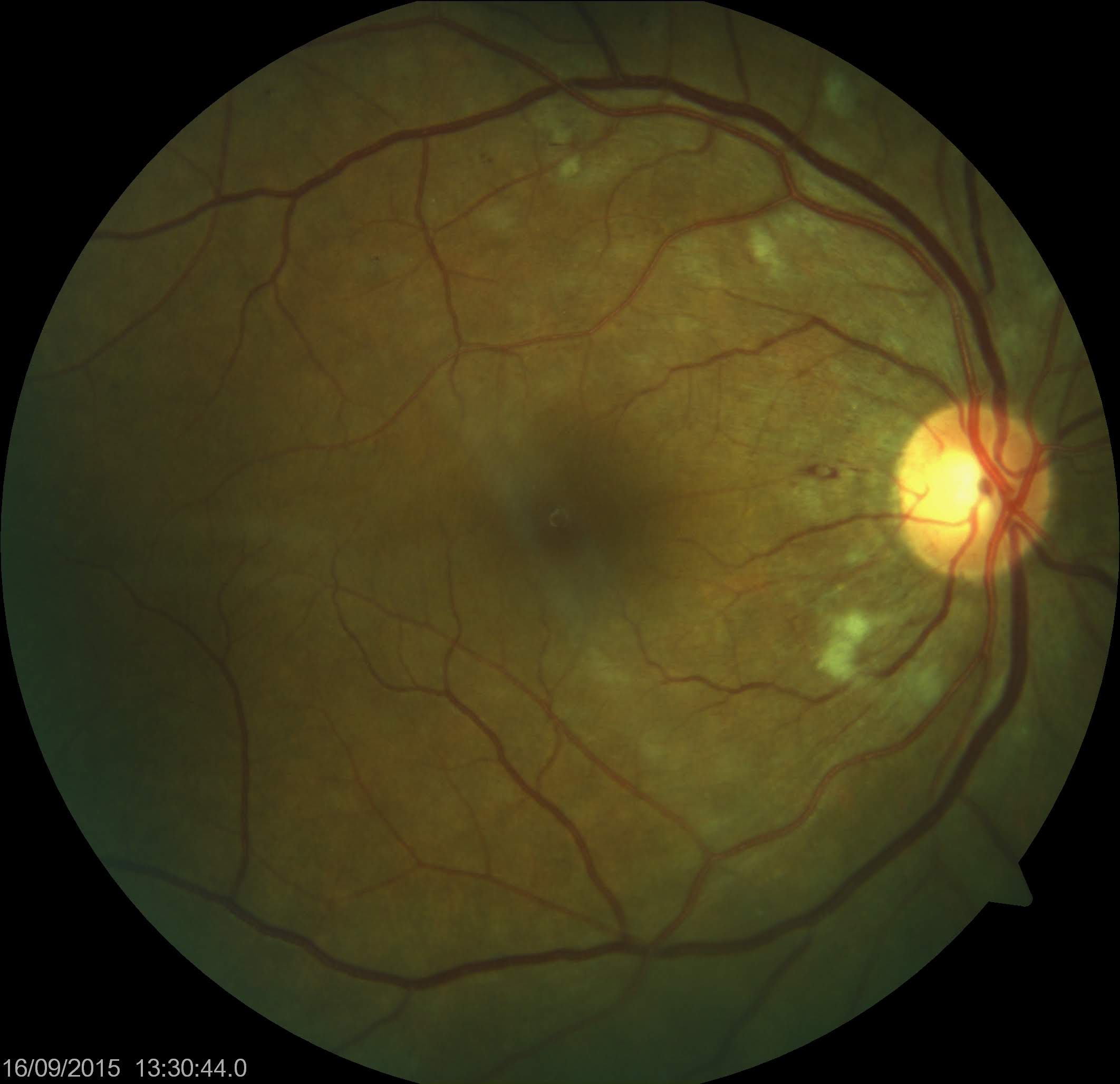
In 1986, the Food and Drug Administration (FDA) approved the use of interferon-α-2a and interferon-α-2b for treating hairy cell leukemia. Since then, the application of interferons has broadened to encompass a variety of conditions. Despite their efficacy, interferon-α treatment may lead to systemic toxicity affecting the central nervous, gastrointestinal, endocrine, cardiovascular, renal, and musculoskeletal systems.[1] The first reported case of ocular toxicity associated with interferon therapy was obtained from Ikebe and associates in 1990. They documented a 39-year-old patient who developed retinal hemorrhages and cotton wool spots after receiving intravenous interferon (see Image. Cotton Wool Spots).[2]
Determining whether to continue interferon treatment or opt for a dose reduction can be challenging due to the various manifestations that may arise. These manifestations may be caused by the occlusion of retinal capillaries or other ischemic etiologies due to immune complex deposition. Although less likely, severe adverse effects such as retinal artery and vein occlusion and optic neuritis are possible.
Etiology
Interferons constitute a family of proteins released by various cells in response to viral infections. They are crucial in promoting innate and acquired immune responses to restrict viral propagation. In addition, interferons can combat bacterial and parasitic infections, inhibit cell division, and promote or impede the differentiation of cells. The 3 main classes of interferons are interferon-α, interferon-β, and interferon-γ. These interferons activate natural killer cells and macrophages and also increase host defenses. They achieve this by upregulating antigen presentation through an increase in the expression of major histocompatibility complex antigens. Following secretion, interferons bind to receptors on neighboring cells, prompting the production of antiviral proteins and additional interferons.
Epidemiology
According to various studies, the reported incidence of interferon-induced retinopathy ranges from 18% to 86%.[3] Higher incidences are observed in certain regions of Japan, ranging between 50% and 86%.[4][5] Increased rates of retinopathy appear in older adults and those with systemic conditions such as hypertension and diabetes. The likelihood of retinopathy is also thought to be related to the dose of interferon.[5]
Interferon-induced retinopathy may occur between 2 and 28 weeks of initial treatment, usually within the first 3 months of therapy.[5] Patients who are nonresponsive to interferon therapy appear to have a higher incidence of interferon-induced retinopathy.[6] Notably, the incidence of retinopathy was higher in studies with a more rigorous schedule of ophthalmic evaluation.[4]
As interferon-induced retinopathy is usually a transient disorder that may resolve spontaneously over weeks to years, extending intervals between ophthalmology examinations could lead to missing some cases. Therefore, studies with less frequent eye examinations, particularly in the initial 6 months of interferon therapy, may report a lower incidence.[7][8] Furthermore, studies initiating ophthalmic evaluation only after the patient becomes symptomatic may result in an underreported incidence.[9][10] To date, researchers are uncertain about the factors determining which individuals will experience interferon-induced retinopathy and who will develop mild versus severe vision loss.
Pathophysiology
The exact pathogenesis of the development of interferon-induced retinopathy is unclear. Some authors suggest that immune complex deposition causes the occlusion of retinal capillaries and results in the formation of cotton wool spots. Another potential mechanism could involve an ischemic insult. Additional suggestions include immunological dysfunction and increased adhesion of activated leukocytes to vascular walls.
Factors Supporting Ischemic Insult as the Cause of Interferon-Induced Retinopathy
Cotton wool spots are focal areas of retinal capillary nonperfusion caused by the obstruction of a retinal arteriole and the resulting ischemia. Local ischemia induces swelling of ganglion cell axons by damaging axoplasmic transport. This results in retinal nerve fiber layer infarcts and the deposition of intra-axonal organelles.[11][12] The accumulation of neuronal debris resulting from local ischemia gives rise to cotton wool spots. The presence of cotton wool spots, retinal capillary nonperfusion on fluorescein angiogram, and retinal vascular occlusion supports ischemic pathogenesis of interferon-induced retinopathy.[11][13] Another study indicates that interferon increases retinal blood velocity, wall shear rate, and retinal blood flow.[14] These effects can lead to endothelial dysfunction, platelet aggregation, and adherence of white blood corpuscles to the endothelium.[15]
Factors Supporting Immune Complex Deposition as the Cause of Interferon-Induced Retinopathy
Deposits of immune complexes in blood vessel walls lead to reduced retinal capillary perfusion and cotton wool spot formation, which may also contribute to the pathophysiology of interferon-induced retinopathy.[16][17] The deposition of inflammatory cytokines in the blood vessel wall may be the catalyst. Interferon-α can potentially induce prothrombotic autoantibodies and elevate the production of complement 5a—a potent platelet aggregator.[18]
Some studies show an association between interferon-induced retinopathy and elevated vascular endothelial growth factor (VEGF) serum levels.[10] Although VEGF is associated with neovascularization, the correlation between interferon-induced retinopathy and high serum VEGF levels remains unclear. Animal studies suggest a potential antiangiogenic effect of interferon.[19]
According to a study conducted by Abe and associates about the long-term effect of interferon on mice, it was demonstrated that mice pretreated with urethane showed occlusion of the retinal vascular bed. In contrast, those without pretreatment did not show this effect.[5] The researchers concluded that a preexisting diseased retina is necessary to develop interferon-associated retinopathy.[20]
Potential Risk Factors Leading to the Development of Interferon-Induced Retinopathy
Systemic hypertension: Retinopathy caused by systemic hypertension can be considered a differential diagnosis for interferon-induced retinopathy. The presence of systemic hypertension may elevate the risk of interferon-induced retinopathy.[5]
Diabetes: Interferon-induced retinopathy is often found in patients with diabetes or diabetic retinopathy, which can cause microvascular damage.[21] Interferon has the potential to aggravate diabetic retinopathy.[5] Uncontrolled diabetes may contribute to the development, intensify the severity, and promote the progression of interferon-induced retinopathy.
Patient age: Older adults are more vulnerable and at a higher risk of developing interferon-induced retinopathy.
Interferon dose: Clinicians typically administer interferon using a high-loading or induction dose, followed by a maintenance or tapered dose. The detection of interferon-induced retinopathy usually occurs within 3 months of the initial loading or induction therapy. Higher doses and a greater frequency of administration per week may predispose individuals to interferon-induced retinopathy.[5] A trial investigating the use of interferon-α-2a for patients with choroidal neovascularization secondary to age-related macular degeneration revealed that the incidence of interferon-induced retinopathy was dose-dependent.[22]
Concomitant medications [23]
Arterial sclerosis: Diabetes and systemic hypertension are the 2 most common and clinically significant risk factors for the development of interferon-induced retinopathy.[2]
History and Physical
Reduced visual acuity, floaters, fluctuations in vision, and the perception of dark spots in the vision constitute the main complaints during symptomatic episodes. Although retinal hemorrhages and cotton wool spots may be observed in clinical findings, most patients remain asymptomatic. Retinal hemorrhages and cotton wool spots may occur independently or concurrently, typically localizing in the posterior pole, particularly around the optic disc.[5][24]
Optic disc hyperemia and macular edema may also be observed as additional findings, and these manifestations can occur unilaterally or bilaterally. Retinal hemorrhages are usually superficial and appear as flame-shaped marks, sometimes with a white center. In the presence of retinal microaneurysms, it is essential to exclude coexisting diabetes.[25] Many patients with interferon-associated retinopathy may also have coexisting diabetes or hypertension.
Although less common, some additional optic adverse effects that may occur are central retinal artery occlusion (CRAO), central retinal vein occlusion (CRVO), combined CRAO and CRVO, CRVO with branch retinal arterial occlusion, macular edema, non-arteritis anterior ischemic optic neuropathy, optic neuritis, optic disc edema, neovascular glaucoma, vitreous hemorrhage, preretinal hemorrhage, Vogt-Koyanagi-Harada disease, ocular toxocariasis, ocular sarcoidosis, retinal detachment, subconjunctival hemorrhage, choroidal neovascular membrane, uveitis, oculomotor nerve palsy, ocular myasthenia, trichomegaly of the eyelashes, and panophthalmitis.[5][26]
Common to these conditions are severe and permanent visual impairment, which persists despite discontinuing interferon therapy and implementing appropriate ophthalmological management. Some authors believe that although interferon may not be entirely responsible for these complications, it could be related to preexisting ocular pathology. As most atypical presentations are case reports, these occurrences may be coincidental and unrelated to interferon therapy. Although many authors assert that there is no difference in the clinical features between patients receiving pegylated interferon and standard interferon,[5] a study observed that the number of patients with interferon-associated retinopathy was more than twice as high with pegylated interferon-α compared to standard interferon-α.[27]
Evaluation
Any patient complaining of ocular symptoms during interferon treatment necessitates a comprehensive examination. Special attention should be given to older adults, those receiving higher interferon doses or in combination with other medications, and those with preexisting conditions such as hypertension and diabetes. Visual acuity testing, pupil examination, and a dilated fundal examination are mandatory. Fluorescein angiography may reveal areas of capillary dropout, hemorrhages, and cotton wool spots that cause blocked fluorescence. Optical coherence tomography is useful for diagnosing macular edema, particularly in subtle cases.[1][28][29]
The FDA labeling for interferon-α-2b recommends a dilated eye examination for all patients before starting treatment. However, certain authors debate the need for a baseline eye examination, as the clear benefit of getting one has yet to be established.[2] Any preexisting retinopathy in patients should be ruled out. Patients with preexisting retinopathy should be screened monthly during interferon therapy. Asymptomatic individuals or those without preexisting retinopathy should undergo eye examinations every 3 months unless their visual acuity declines.
Treatment / Management
If a patient experiences an ophthalmological complication during interferon therapy, the general recommendation is to discontinue interferon treatment. Fundal findings typically exhibit rapid improvement upon discontinuation of interferon therapy. Interestingly, retinal findings can resolve spontaneously despite continuing interferon without requiring a dose reduction.[5][27] Consequently, some authors propose discontinuing treatment only in cases of severe retinopathy, visual decline, other ocular symptoms, or in individuals with preexisting conditions such as diabetes.
Dose reduction of interferon is an alternative strategy for patients with interferon-associated retinopathy. However, reducing the interferon dose increases the risk of treatment failure and exacerbation of the primary disease. Therefore, the decision to discontinue or reduce the interferon dose is made after a comprehensive evaluation of the risks and benefits, considering the prescribing information provided by the manufacturer.
Differential Diagnosis
The differential diagnoses of interferon-induced retinopathy include hypertensive retinopathy, ocular ischemia, Lupus retinopathy, HIV retinopathy, Purtscher retinopathy, Purtscher-like retinopathy, and retinopathy from leukemia or lymphoma.[30]
The hepatitis C virus itself can cause retinopathy, which manifests as retinal cotton wool spots and retinal hemorrhages in patients with chronic hepatitis C. The proposed risk factors for this condition include chronicity of hepatitis, thrombocytopenia, advanced age, systemic hypertension, female gender, and associated hepatic cirrhosis. Retinal findings were observed before initiating interferon treatment, and the retinopathy tends to exacerbate following interferon therapy. Additional research is required to evaluate the role of associated systemic diseases and these retinal changes.[31]
Prognosis
The prognosis for interferon-induced retinopathy is generally favorable, with most cases resolving upon discontinuation of interferon. Spontaneous resolution during interferon therapy is common, often eliminating the need for dose reduction.[2] Visual outcomes are usually excellent, and many patients remain asymptomatic. Therefore, regular screening examinations are crucial for timely detection of interferon-induced retinopathy.
Some cases of interferon-induced retinopathy do not resolve, and rare instances of poor visual outcomes in typical interferon-induced retinopathy can occur.[25][32][33] According to a review, interferon-induced retinopathy caused a visual deficit in 1 among 1289 patients.[2] The visual outcomes associated with the atypical ocular complications of interferon therapy are usually unfavorable, often resulting in vision loss.
Complications
The primary complication of interferon-induced retinopathy is vision loss. Although most cases are mild and reversible, severe vision loss can occur in certain cases. Additional possibilities include retinal artery and vein occlusions, neovascular glaucoma, and macular edema.[13][34]
Deterrence and Patient Education
Interferon therapy, commonly used for various medical conditions, carries the potential risk of interferon-induced retinopathy. This condition damages small blood vessels in the retina, resulting in decreased visual acuity and, in severe cases, blindness. Patients with interferon-induced retinopathy are often asymptomatic, and the damage may resolve upon discontinuation of interferon or spontaneously, despite continuing treatment. However, the risk of vision loss persists. Retinal examination using an ophthalmoscope can reveal signs of retinopathy, including cotton wool spots, retinal hemorrhages, and macular edema.
Patients must undergo a thorough eye examination before starting interferon therapy and continue with regular follow-up eye exams. An initial examination helps to establish a baseline for the patient's retina, preventing the initiation of interferon in individuals with preexisting retinopathy, such as those with diabetes or hypertension. Maintaining optimal control of blood glucose levels and blood pressure is crucial, as both conditions can contribute to retinopathy.
In severe cases, interferon therapy may cause optic neuropathy and occlusion of major retinal veins and arteries, leading to vision loss and necessitating immediate attention. Patients on interferon should promptly report any visual changes or other eye symptoms to their healthcare professional. Although some patients may continue therapy without progression, most experience complete resolution after discontinuation.
Enhancing Healthcare Team Outcomes
Patients undergoing interferon therapy are at risk of interferon-induced retinopathy induced by interferon, which highlights the crucial requirement for early identification and prevention to mitigate morbidity and mortality. Collaboration among healthcare professionals, including physicians, advanced practice practitioners, nurses, and pharmacists, is necessary to effectively care for patients and manage potential adverse effects such as retinopathy. Clinicians must possess clinical skills and competence to prevent and control possible adverse effects such as interferon-induced retinopathy.
The healthcare team should ensure regular screening for retinopathy from the beginning of treatment and at intervals throughout. Strict glycemic and blood pressure control is crucial for those with diabetes and hypertension. Patients need education on potential retinopathy symptoms and should know when to promptly report any visual changes. Interprofessional communication is vital among clinicians to ensure well-controlled comorbid illnesses and timely access to the latest laboratory evaluations and eye examination results. This coordinated effort significantly reduces the risk of vision loss associated with interferon-induced retinopathy and contributes to an overall reduction in morbidity and mortality.