Continuing Education Activity
Anesthetic vaporizers are an essential piece of anesthesia equipment. This activity reviews the modern anesthetic vaporizer classification, the physics principles behind their functioning, and possible hazards that could be present at the time of using an anesthetic vaporizer. It also highlights the role of the interprofessional team in managing the technical complications related to the use of anesthetic vaporizers in patients undergoing general anesthesia.
Objectives:
- Identify the indications and contraindications for the use of anesthesia vaporizers.
- Describe the equipment required to administer anesthesia vaporizers.
- Review the potential complications and clinical significance of anesthetic vaporizers.
- Outline interprofessional team strategies for improving care coordination and communication to advance the use of anesthesia vaporizers and improve clinical outcomes.
Introduction
Since the first publicly performed anesthetic procedure at Massachusetts General Hospital in 1846, vaporizers have been an essential component of anesthesia equipment.[1] The emergence of potent inhalational anesthetics with unique properties has influenced the evolution of vaporizers. Modern anesthetic vaporizers have been developed to provide accurate amounts of anesthetic gas while mitigating the effects of temperature and barometric pressure on the evaporation process, allowing anesthesiologists to conduct their work with greater safety.
The functioning of vaporizers is complex; and requires an in-depth understanding of thermodynamics, gases, and physics. While engineering may not be the forte of many anesthesiologists, familiarity with the proper functioning of the anesthetic equipment and the ability to recognize equipment failures are essential to decrease the potential for patient hazards.[2]
Anatomy and Physiology
Rather than providing a comprehensive review of the physics that dictates vaporizer functioning, this section will address key concepts that all anesthesiologists should understand when using these devices.
By definition, vapor is the gaseous phase of a substance. To reach this phase, a substance must reach a temperature below its critical temperature, which is different for every drug. The behavior of a gas in a sealed container is explained by the ideal gas law, which states that the pressure exerted by the gas on the walls of the container is directly proportional to the number of gas molecules (n) and the temperature inside the container expressed in degrees Kelvin (K). However, it is inversely proportional to the volume (v) of the container. In other words, gas molecules inside the container behave like points in space colliding with each other and the container walls, generating pressure. Under normal operating conditions, anesthetic gases will behave as ideal gases.
Expressing Gas Concentrations and Minimum Alveolar Concentration
Quantifying the proportion of an individual gas within a mixture of gases can be achieved by calculating either its partial pressure (Dalton law) or the percentage of volume (V/V%) occupied by this particular gas in comparison to the mixture.
Percentage of volume (V/V%) of oxygen = Partial pressure of oxygen / Total pressure of air * 100
- V/V% of isoflurane = (239 mmHg / 760 mmHg) * 100 = 31%
The minimum alveolar concentration (MAC) is defined as the concentration of anesthetic gas that prevents movement from the surgical stimulus in 50% of patients at 1 atmosphere of pressure (760 mmHg).[3] It is expressed in volume percent. Although the MAC can be affected by several variables such as age and barometric pressure, this value is still clinically useful considering vaporizer control knobs are calibrated and marked in terms of anesthetic concentration. The minimal alveolar partial pressure (MAPP) is the corresponding partial pressure (mmHg) for each MAC (concentration) value. MAPP is important, bearing in mind that the partial pressure in the brain is the final determinant of the anesthetic gas effect and, consequently, its potency.
Duration (or consumption rate) of the anesthetic liquid inside of vaporizers can be calculated, giving more of a clinical application to this concept. One milliliter of anesthetic liquid is able to produce up to 210 ml of anesthetic gas, though this varies between drugs. Based on this calculation, the following formula can be applied:
- Ml of liquid anesthetic/hour = 3 (or 3.3 if is sevoflurane or 2.85 if is desflurane) * Dial concentration (V/V%)* fresh gas flow (Liters/minutes)
Example:
How many milliliters of sevoflurane would be consumed if providing 2.5% V/V% and a fresh gas flow of 3 liters?
Ml of sevoflurane= 3.3 * 2.5% * 3 liters/minute= 24.7 ml/hour of sevoflurane consumption
Saturated Vapor Pressure (SVP)
Evaporation is the phenomenon by which molecules in the liquid phase (with high kinetic energy) escape from the surface of a liquid to enter the gas phase. Inside the vaporizer, molecules will enter the gas phase until the rate of vaporized molecules equals the rate of molecules returning to the liquid phase. This dynamic equilibrium is known as saturation. The pressure exerted by this gas is known as “saturated vapor pressure.” SVP increases in a non-linear fashion as the temperature of an anesthetic in the liquid phase increases and is independent of the barometric pressure. The SVP for all anesthetic gases is measured at 20ºC and is a unique characteristic for each drug. For example, isoflurane has an SVP of 239 mmHg.
Evaporation is an energy-consuming process that can be expressed in terms of heat. The amount of energy required to convert 1 gram of liquid into vapor at a constant temperature is called the “latent heat of vaporization.” Thus, a liquid at normal temperature evaporates and loses temperature (cooling), which leads to a reduction of vapor pressure. Modern anesthetic vaporizers mitigate and compensate for evaporative cooling, preventing a decrease in the output of anesthetic gas.[4]
Boiling Point
The boiling point is defined as the temperature at which vapor pressure equals atmospheric pressure, and a substance changes from the liquid to the gas phase, commonly expressed in degrees centigrade. The boiling point is 1 atmosphere for anesthetic gases. It is especially important to note that desflurane has a boiling point of 22.8ºC. As the boiling temperature can be affected by the barometric pressure, changes in altitude can affect the performance of desflurane vaporizers. However, this is no longer a problem since the introduction of the Tec-6 vaporizer.
Specific Heat and Thermal Conductivity
Specific heat is the number of calories required to increase the temperature of 1 gram of a substance by 1ºC. Thermal conductivity describes how well any material allows heat to flow through it. Vaporizers are designed with materials of high specific heat and thermal conductivity, allowing them to resist the changes of external and internal (cooling) temperature. Thus, a constant temperature is maintained inside the vaporization chamber during the evaporation process.
Two mechanisms for preventing heat loss have been widely used in vaporizer fabrication. Old vaporizers use a “heat sink” like glycol or water, while modern vaporizers are covered with a dense metal that prevents heat loss, such as copper. However, maintaining a gas concentration at a lower temperature requires more gas to pass through the vaporization chamber. Temperature-compensated devices have been manufactured for this purpose. These devices use a bimetallic strip composed of two metal bands with different coefficients of expansion that bend according to the temperature inside the vaporization chamber. If the temperature decreases, the strip allows the fresh gas flow to enter the vaporization chamber. In modern models, this strip can be found in the bypass chamber to prevent corrosion of the piece. Older vaporizers are not temperature-compensated. Another common piece used for temperature compensation is the aneroid bellows. These are attached by a rod to a cone that is located in the orifice of the bypass chamber. When the temperature decreases, the bellows contract and make the cone partially obstruct the bypass channel, increasing fresh gas flow to the vaporization chamber.
Classification of Modern Vaporizers
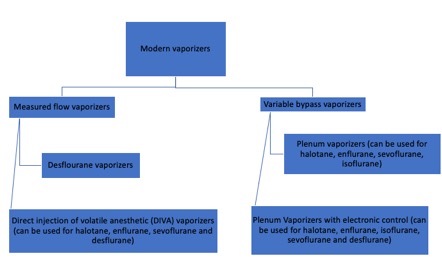
Modern vaporizers are designed to provide an accurate and adjustable concentration of anesthetic gas to the patient. The concentration of anesthetic gas inside the vaporizer is extremely high and must be diluted. Dorsch and Dorsch (1979) proposed a vaporizer classification, though current advances in technology have made this classification less reliable. Additionally, a vaporizer can share only one to several of the characteristics in their classification. A better explanation of modern vaporizers can be obtained by separating them into two groups, according to the input of fresh gas flow (Figure 1).
Mixing the Gases
As mentioned previously, V/V% is the percentage of the volume occupied by a particular gas within a mixture of gases. When the V/V% in the vaporization chamber is too high, it can be diluted in two ways. The first is by splitting the fresh gas flow such that a small portion of gas can enter the vaporization chamber, and the rest bypass it.[5] The portion of gas entering the chamber will vary depending on the type of anesthetics gas and the V/V% of the dial. This is the major mechanism of variable bypass vaporizers. The second way to dilute the anesthetic gas is by injecting the vaporized anesthetic directly into the fresh gas flow in the absence of splitting. This is the mechanism of measured flow vaporizers.[6]
Indications
Anesthetic vaporizer use is indicated in every surgery that requires general anesthesia with inhalation anesthesia technique.
Contraindications
In case of suspected incorrect functioning of the vaporizer, it should be removed and replaced. In patients with the predisposition of malignant hyperthermia, the vaporizers must be removed from the anesthetic machine, and intravenous anesthesia would be the best option in those cases.[7]
Equipment
Variable Bypass Vaporizers
Variable bypass vaporizers can be divided into two categories: Draw over and plenum (with or without electronic control). In draw over vaporizers, the carrier gas is drawn into the vaporization chamber, where it passes over anesthetic liquid. Negative pressure (sub-atmospheric pressure) is provided directly from the patient breathing, which draws the anesthetic gas from the vaporizer to the patient. Plenum vaporizers are characterized by a pressurized vaporization chamber that generates an increased internal resistance (above atmospheric pressure) for fresh gas flow. This type of vaporizer is currently the most commonly used and is characterized by its accuracy in the mixture of gases provided to the patient.[8]
Mode of Operation
Once the fresh gas leaves the flowmeter and enters the vaporizer, it is divided into two streams. One stream flows through the bypass circuit and the temperature compensating assembly. The other stream flows downward through the pressure compensating labyrinth into the vaporizing chamber, where it is mixed with the vapor of the liquid anesthetic drug. It then passes through the concentration cone, where it is mixed again with the fresh gas flow. The temperature compensating assembly adjusts the ratio of bypass flow to the vaporizing chamber, compensating for changes in anesthetic vapor pressure secondary to temperatures changes.
Main Characteristics of Variable Bypass Vaporizers
The dial release can be used to control the interlock mechanism, pressed inward toward the dial while the vaporizer lever is in the locked position. Using the interlock mechanism allows the port valves of the manifold to open and the vaporizer to operate. The interlock mechanism also prevents adjacent vaporizers from being switched on. These vaporizers are calibrated up to 8% concentration of sevoflurane and for other anesthetic drugs up to 5% concentration. These can be filled with 300 ml of dry wicks, of which 75 ml are retained as the minimum level of filling in the wicks system.
Cassette Type Vaporizer
This vaporizer can be used with certain models of anesthesia machines, with halothane, isoflurane, enflurane, sevoflurane, and desflurane. The unit is composed of two parts. The first part is permanently housed in the anesthetic machine, and the second is an interchangeable cassette that contains the anesthetic liquid and acts as a vaporization chamber. It is important to note that this vaporizer is controlled by a microprocessor that receives information from the flowmeters about the composition of the gas, from temperature and pressure sensors inside the vaporization chamber, and from the output of the vaporization chamber, with the purpose of providing an accurate concentration of anesthetic gas. Additionally, this vaporizer has a unidirectional valve that prevents retrograde flow to the variable flow chamber. The cassette vaporizer also has electronic control of oxygen concentration, warranting that an oxygen concentration of less than 25%, independent of the anesthetic gas concentration, will never be delivered to the patient. In case the pressure inside the cassette exceeds 2.5 bars (1899 mmHg), this vaporizer has a pressure release valve that will open, preventing this pressure from being transmitted to the respiratory circuit. Finally, this vaporizer is unique, considering that tipping is not a problem for this device.
Factors Affecting the Performance of Variable Bypass Vaporizers
Fresh Gas Flow Effect
Extreme flows (more than 15 L/min or less than 250 ml/min) can decrease the final anesthetic gas concentration. When the flow is extremely low, enough air turbulence is not generated to move the anesthetic gas particles, delivering a lower anesthetic gas concentration than marked on the dial. When the fresh gas flow is too high, the anesthetic gas concentration also decreases compared with what is marked in the dial. The latter scenario could be multifactorial due to faster evaporation, incomplete saturation of the mixture of gases, or high resistance of the vaporizing chamber.
Effect of Temperature Changes
As mentioned above, temperature changes can increase or decrease the amount of vapor generated in the vaporization chamber. However, this concentration is stable in modern vaporizers within a big range of temperatures. This variable could be affected by an anesthetic liquid reaching its boiling point inside the vaporizer. In this situation, the temperature compensation system will not be enough to control the gas evaporation. Though this is an extreme and improbable scenario, halogenated drugs like isoflurane and halothane are susceptible to this problem at higher altitudes, where the boiling point is lower. In fact, the user’s manual of some vaporizers limits the use of halothane and isoflurane to altitudes of more than 1450 to 3000 meters above sea level. Most vaporizers are built to work under temperatures of 10°C to 40ºC, though this varies according to the manufacturer.
Composition of the Carrier Gas
Although this effect is not clinically significant, the viscosity of air and nitrous oxide is lower (more soluble) than that of oxygen. As nitrous oxide is diluted in anesthetic fluid, the production of anesthetic gas decreases. Furthermore, splitting of the carrier gas decreases flow through the vaporization chamber, so a reduction in output is expected, especially with nitrous oxide.
Pumping Effects
This describes the effect of the transmission of retrograde pressure from the patient circuit to the vaporizer during the inspiratory phase with positive pressure or during the use of an oxygen flush valve. Molecules of anesthetic gas will compress in the vaporization and variable flow chambers. When pressure is released during the expiratory phase, vapor will exit the chamber through the normal output track and additionally from the input track of the chamber. In this way, the final concentration of anesthetic gas will be higher. This effect is greater when the low flow of fresh gas is used, and the liquid levels of the anesthetic gas in the vaporizer are low. Modern anesthetic machines have unidirectional valves in the inspiratory and expiratory arms to prevent this effect.
Measured Flow Vaporizers
These vaporizers are characterized by a separated stream for anesthetic vapor carrying that is then mixed with the fresh flow gas. Some of these devices have been specially created for desflurane for two reasons. The first is that this halogenated anesthetic has a really high saturated vapor pressure (669 mmHg at 20ºC). A conventional vaporizer would need a flow of 73 L/min to deliver a concentration of 1% desflurane to the patient, in contrast to the 5 L/min or less that is needed for other halogenated drugs. Second, desflurane boils at almost room temperature (22.8ºC), losing heat in absence of temperature compensation. However, temperatures may vary in different operating rooms. Intermittent boiling will result in large fluctuations of anesthetic gas delivery due to unpredictable saturated vapor pressure.[9]
Mode of Operation
Desflurane vaporizers work similarly to a blender of gases. These vaporizers have two independent gas circuits aligned in parallel. The fresh gas circuit passes through a fixed restrictor and leaves the vaporizer through the common gas outlet. The anesthetic gas circuit is originated in the sump, which works as a desflurane vapor reservoir. The sump is heated at 39ºC, a temperature that exceeds the boiling point of desflurane and generates up to 1300 mmHg of vapor pressure (2 atm). Downstream from the sump, there is a shut-off valve that opens once the vaporizer warms up, and the concentration dial is open. Downstream from the shut-off valve, there is a variable restrictor that can be controlled by the user and adjust the concentration of anesthetic gas. Both circuits finally join before leaving the vaporizer together form one flow to the patient. Despite the fact that these circuits are physically separated, they are interconnected electronically through a differential pressure transducer, a pressure-regulating valve, and a control electronic system. The backpressure generated by the pass of the fresh gas flow through the fix restrictor is proportional to the flow rate that is sensed by the differential pressure transducer. The difference of pressures between the fresh gas flow circuit and the desflurane circuit is calculated and transmitted to the control electronic system. This system tunes the pressure regulating valve equalizing the pressure of the two circuits. This equalized pressure is known as working pressure, which is constant at an FGF rate.
The mixture of anesthetic produced by the vaporizer is constant because the fresh gas flow and the anesthetic gas flow are always proportional; this is different from the variable bypass vaporizers.
Factors Affecting the Performance of Desflurane Vaporizers
Barometric Pressure
The desflurane vaporizer works more like a gas blender than a vaporizer. Despite this device is able to maintain a constant volume percent (V/V%) output, the partial pressure of anesthetic gas decreases in the same way as the barometric pressure decreases. This is an important fact to have in mind since the partial pressure of the anesthetic in the brain will determine the anesthetic depth.
Carrier Gas Composition
Nitrous oxide has a decreased viscosity that is not able to generate enough working pressure inside the desflurane vaporizer. Thus, at low flow rates of nitrous oxide, the concentration of anesthetic gas delivered to the patient will be decreased by up to 20% from the value marked in the dial.[10]
Complications
Safety Characteristics and Hazards of Vaporizers
It is typically rare to have problems of any kind with anesthetic vaporizers, due to the huge engineering efforts for manufacturing these masterpieces. Many of the hazards related to anesthetic vaporizers are preventable and are more so related to inadequate manipulation by the provider.[11]
Incorrect Agent Filling
Many modern vaporizers have agent-specific inputs, making incorrect filling a user error. Vaporizers are, therefore labeled with colors to help reduce the probability of incorrect filling. The consequences of filling the vaporizer with the wrong anesthetic agent include overdose, as could be observed with desflurane, or underdose which could occur with the other halogenated liquids.[12]
Tipping
Inclination of a variable bypass vaporizer more than 45º during transportation or replacement can cause the anesthetic liquid to flow into the variable bypass chamber. Once the dial is open, anesthetic liquid in the bypass chamber increases the concentration of anesthetic gas beyond what is marked on the dial. This situation is particularly dangerous for the patient and can last for an amount of time that is difficult to predict. The solution is to increase vaporization in the bypass chamber by decreasing the concentration marked on the dial and simultaneously increase the FGF to 10 L/min for 20-30 minutes.[13]
Simultaneous Administration of More Than One Vapor
Since newer anesthetic machines with interlock safety devices do not allow the user to open two dials at the same time, this issue is somewhat anecdotal. Some older modules allow users to remove the central vaporizer, deactivating the interlock mechanism and permitting administration of more than one vapor at a time.
Overfilling the Vaporizer
Newer vaporizers have a hole at the appropriate level to prevent overfilling. However, older vaporizers can still be overfilled. The excess anesthetic liquid will flow into the bypass chamber and, once the dial is open, potentially increase the concentration of anesthetic gas for an unpredictable amount of time. There is also a case report of vaporizer malfunctioning secondary to overfilling where the wicks of the vaporizer were completely covered by the anesthetic liquid, preventing appropriate evaporation.[14]
Vapor Leak into the Fresh Gas Line
The size of the leak depends on the ambient temperature and configuration of internal ports. Leakage is usually too small to produce a clinical effect. However, it can trigger an episode of malignant hyperthermia in patients with a known or unknown predisposition.
Leaks
The majority of gas leaks will be identified by checking the low-pressure system before using the anesthetic machine with a patient, and sometimes all that is needed is tightening a loose filler cap. The most concerning consequence of an unknown leakage is the administration of light anesthesia. Several case reports exist reporting this issue, with most of them having in common the inappropriate positioning of vaporizers on the back bar of the anesthesia machine. Others report inappropriate adjustment in the mounting system due to a missing rubber O-ring. Additionally, incompatibility between vaporizers and interlocks from different manufacturers could be the cause of leakage. Timely identification of this problem is facilitated by checking the anesthetic machine with the vaporizer in the ON and OFF positions.[15]
Clinical Significance
Vaporizers are an important part of anesthesia equipment. An understanding of physiology and physics is warranted to ensure the adequate use of these devices. The timely recognition of problems in vaporizer functioning is also essential, as this will decrease the amount of time that patients are potentially exposed to light anesthesia and pain.
Enhancing Healthcare Team Outcomes
Anesthesia vaporizers are an essential part of anesthesia equipment. Understanding physiology, physics, and mechanics behind anesthetic vaporizers ensures the proper use of these devices. The proper functioning and maintenance of these devices requires interprofessional communication between physicians, anesthesia technicians, and certified registered nurse anesthetist (CRNA) to enhance the safety of patients undergoing general anesthesia. [Level 3]