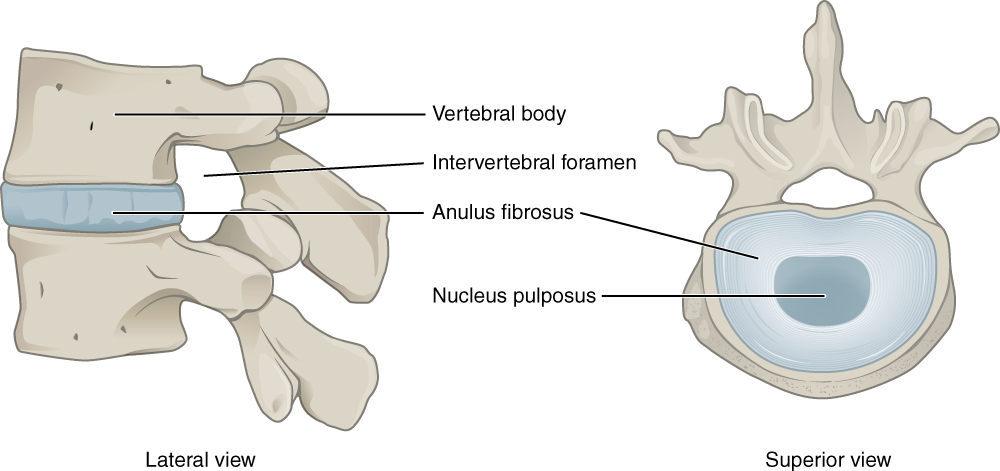
[3]
Nosikova YS, Santerre JP, Grynpas M, Gibson G, Kandel RA. Characterization of the annulus fibrosus-vertebral body interface: identification of new structural features. Journal of anatomy. 2012 Dec:221(6):577-89. doi: 10.1111/j.1469-7580.2012.01537.x. Epub 2012 Jul 3
[PubMed PMID: 22747710]
[4]
Moon SM, Yoder JH, Wright AC, Smith LJ, Vresilovic EJ, Elliott DM. Evaluation of intervertebral disc cartilaginous endplate structure using magnetic resonance imaging. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2013 Aug:22(8):1820-8. doi: 10.1007/s00586-013-2798-1. Epub 2013 May 15
[PubMed PMID: 23674162]
[5]
Chen S, Fu P, Wu H, Pei M. Meniscus, articular cartilage and nucleus pulposus: a comparative review of cartilage-like tissues in anatomy, development and function. Cell and tissue research. 2017 Oct:370(1):53-70. doi: 10.1007/s00441-017-2613-0. Epub 2017 Apr 17
[PubMed PMID: 28413859]
Level 2 (mid-level) evidence
[6]
Nerurkar NL, Elliott DM, Mauck RL. Mechanical design criteria for intervertebral disc tissue engineering. Journal of biomechanics. 2010 Apr 19:43(6):1017-30. doi: 10.1016/j.jbiomech.2009.12.001. Epub 2010 Jan 18
[PubMed PMID: 20080239]
[7]
Rodrigues-Pinto R, Richardson SM, Hoyland JA. An understanding of intervertebral disc development, maturation and cell phenotype provides clues to direct cell-based tissue regeneration therapies for disc degeneration. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2014 Sep:23(9):1803-14. doi: 10.1007/s00586-014-3305-z. Epub 2014 Apr 29
[PubMed PMID: 24777668]
Level 3 (low-level) evidence
[8]
McCann MR, Tamplin OJ, Rossant J, Séguin CA. Tracing notochord-derived cells using a Noto-cre mouse: implications for intervertebral disc development. Disease models & mechanisms. 2012 Jan:5(1):73-82. doi: 10.1242/dmm.008128. Epub 2011 Oct 25
[PubMed PMID: 22028328]
[9]
Raoul S, Faure A, Robert R, Rogez JM, Hamel O, Cuillère P, Le Borgne J. Role of the sinu-vertebral nerve in low back pain and anatomical basis of therapeutic implications. Surgical and radiologic anatomy : SRA. 2003 Feb:24(6):366-71
[PubMed PMID: 12647025]
[11]
Amin RM, Andrade NS, Neuman BJ. Lumbar Disc Herniation. Current reviews in musculoskeletal medicine. 2017 Dec:10(4):507-516. doi: 10.1007/s12178-017-9441-4. Epub
[PubMed PMID: 28980275]
[12]
Nilsson E, Brisby H, Rask K, Hammar I. Mechanical compression and nucleus pulposus application on dorsal root Ganglia differentially modify evoked neuronal activity in the thalamus. BioResearch open access. 2013 Jun:2(3):192-8. doi: 10.1089/biores.2012.0281. Epub
[PubMed PMID: 23741630]
[13]
Ab Aziz CB, Ahmad AH. The role of the thalamus in modulating pain. The Malaysian journal of medical sciences : MJMS. 2006 Jul:13(2):11-8
[PubMed PMID: 22589599]