Continuing Education Activity
The retina is a thin layer of tissue in the posterior segment of the eye near the optic nerve. It contains a layer of photoreceptor cells, and receives focused light from the lens and converts the light into neural signals, because of this, the retina is a vital structure involved in vision and damage to it can have devastating consequences. The macula is the center of the retina and has the highest concentration of rods and cones (photoreceptors). Diabetes can lead to macular edema in a multifactorial manner. This is one of the leading causes of visual impairment in the United States. The first-line treatment usually involves anti-vascular endothelial growth factor (VEGF) therapy; however, new treatments are being developed. This activity outlines the diagnosis, evaluation, and treatment of diabetic macular edema. It also highlights the role of an interprofessional team approach in the management of this condition.
Objectives:
- Describe the implications of diabetic macular edema.
- Review the epidemiology and prevalence of diabetic macular edema.
- Outline treatment strategies for diabetic macular edema.
- Summarize interprofessional team strategies for improving care coordination and communication to advance the management of diabetic macular edema and improve outcomes.
Introduction
The retina is a thin layer of tissue in the posterior segment of the eye near the optic nerve. It contains a layer of photoreceptor cells, and receives focused light from the lens and converts the light into neural signals, because of this, the retina is a vital structure involved in vision and damage to it can have devastating consequences. The macula is the center of the retina and has the highest concentration of rods and cones (photoreceptors). Diabetes can lead to macular edema in a multifactorial manner. This is one of the leading causes of visual impairment in the United States. The first-line treatment usually involves anti-vascular endothelial growth factor (VEGF) therapy; however, new treatments are being developed.[1][2]
Etiology
Diabetes and subsequent hyperglycemia results in the formation of advanced glycation end products (AGEs). The specific etiology of diabetic retinopathy is unclear and likely, the result of many interplaying factors. AGEs are osmotically active, and they may be responsible for fluid accumulation in the macula. Diabetes also results in disruption of the blood-retinal barrier (BRB), and this is likely critical in the pathogenesis of diabetic associated macular edema. AGEs are also associated with increased inflammatory markers such as VEGF, leukocyte adhesion, and protein kinase C.[3][4]
Epidemiology
Diabetic macular edema (DME) is one of the leading causes of vision loss worldwide. It affects over 75,000 Americans yearly, and nearly 100 million people worldwide show some signs of macular edema secondary to diabetes. Some studies have shown that nearly 1 in 3 people with diabetes have some evidence of macular edema. The prevalence of DME is higher in individuals with type 1 diabetes than those with type 2 diabetes. In patients that have been diagnosed with diabetes, the ten-year incidence of DME is approximately 20% in patients that were diagnosed before the age of 30, and approximately 40% in patients diagnosed over the age of 30. Another study found that approximately 27% of patients develop signs of macular edema within 9 years of diabetes onset. Several studies that address different demographics illustrate an increasing incidence of DME.[5][6]
Pathophysiology
The underlying pathophysiology of diabetic macular edema is secondary to the disruption of the BRB. The BRB isolates the photoreceptors of the retina from the ophthalmic vasculature. The BRB functions in a complex manner that involves several factors that work in tandem; however, many of the specific physiologic processes are poorly understood. The BRB involves two major compartments: an outer and inner barrier. Animal models have illustrated that the permeability of both compartments is disrupted after the onset of diabetes. Disruption of this barrier results in the accumulation of macular edema; however, the process is more complicated than this and also involves various inflammatory markers upregulated by AGEs, hyperglycemia, and diabetes. Diabetes also results in vasoconstriction, which upregulates VEGF expression. VEGF also results in macular edema and results in vasculogenesis, which results in further retinal disease.[7][8]
History and Physical
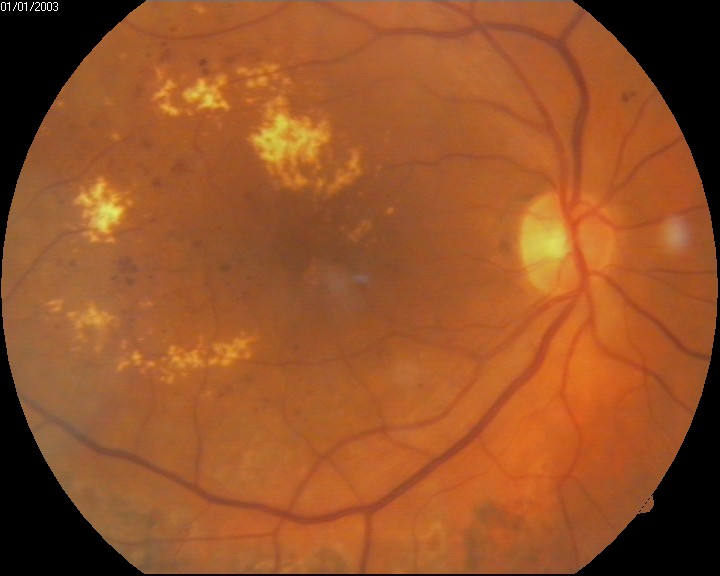
Patients with diabetes receive more frequent ophthalmic evaluations than the average person; usually, patients with diabetes should get eye exams every year. In certain instances, this may be more or less frequent). Diabetic macular edema is diagnosed stereoscopically, and the macula appears thicker with diabetic macular edema when compared to eyes not impacted by DME. The Early Treatment Retinopathy Study (ERDRS) has defined DME as a thickening of the retina or the presence of hard exudates within 1 disc diameter from the center of the macula, and this is the most commonly used definition. There are various patterns of thickening, including focal, multifocal, and diffuse areas. DME is deemed medically significant if it is associated with any of the following: thickening within 500 mm of the center of the macula, the presence of hard exudates within 500 mm of the center of the macular if associated with thickening of the adjacent retinal tissue, or zones of retinal thickening 1 disk area of which part of which is within 1 disk diameter of the macular center. It is important to note that only of these features must be present in order for diabetic macular edema to be deemed as clinically significant, although it is not uncommon for several of these features to be present.[7][9]
Evaluation
The primary evaluation of DME, classically, involves slit lamp evaluation and stereo fundus photography; however, multiple new modalities are being used and developed for the evaluation of diabetic macular edema. Fluorescein angiography (FA) involves the injection of fluorescent dye into the bloodstream. The dye can then be used to detect fluid accumulation qualitatively. Optical coherence tomography (OCT) is a high-resolution imaging modality that has been used to image the retina and detect the thickness of the retina. OCT is less invasive than FA and is subsequently better tolerated. Compared to slit lamp and stereo fundus photography OCT has been more accurate in the evaluation of macular thickness and identifying the location of retinal fluid. The retinal thickness analyzer (RTA) is another imaging modality that is used for assessing retinal thickness. The RTA projects a laser slit beam onto the retina and analyzes the backscattered light with a fundus camera. RTA has been shown to illustrate similar thickness to OCT; however, OCT requires a longer image acquisition time. OCT has better image resolution and is less influenced by ocular media opacities. Both OCT and RTA play an important role in the early detection of DME, while FA is useful in analyzing DME once the diagnosis has been made.[10][11]
Early treatment diabetic retinopathy study criteria for clinically significant macular edema include retinal thickening within 500 µm of the macular center, hard exudates within 500 µm of the macular center with adjacent retinal thickening, or one or more disc diameters of retinal thickening, part of which is within one disc diameter of the macular center.[12]
Treatment / Management
One of the mainstays for diabetic macular edema treatment has historically involved laser photocoagulation, it has been shown to improve visual acuity in a small percentage of patients. The Early Treatment Diabetic Retinopathy Study (EDTRS) provides the guidelines for treatment via laser photocoagulation. Leaking microaneurysms are directly treated, while a combination of focal laser photocoagulation and scatter laser photocoagulation are described as the treatment for DME in certain instances of proliferative diabetic retinopathy and non-proliferative diabetic retinopathy. Although macular laster photocoagulation is a major treatment modality for clinically significant macular edema, it is not curative, and many cases are refractive to the laser therapies. Vitrectomies have been shown to improve DME; however, the science behind this is unclear. With the onset of new research, the clinical guidelines have changed, and current first-line therapy involves anti-VEGF therapy. Other treatment modalities include subthreshold micropulse photocoagulation diode and intravitreal steroid injections.[13][14]
Differential Diagnosis
Macular edema can be caused by several other conditions including but not limited to hypertension, retinal vein occlusion, ruptured microaneurysm, radiation, Irvine-Gass syndrome, and subfoveal choroidal neovascularisation. A good clinical history can help to elucidate the underlying cause of the macular edema. Questions about the patient's onset of diabetes, hemoglobin A1C trending, diet, and presence of other diabetic complications are critical.[13]
Prognosis
The prognosis of diabetic macular edema is contingent upon a few key factors. The most important is the initial presenting severity of the edema: the more severe the initial presentation, the more likely that vision will deteriorate in the future. Treatment, as well as both diabetes and hypertension control, are also critical in preventing the worsening of DME. Given the variance in the condition: between 25% to 30% of eyes with clinically significant macular edema will experience some degree of visual loss within three years.[15]
Complications
The biggest complication with diabetic macular edema is a progressive, irreversible loss of vision; however, various treatment modalities are also associated with complications. Laser photocoagulation is associated with: subretinal fibrosis and scarring, decreased visual acuity and paracentral scotoma, as well as choroidal neovascular membranes. Intravitreal injections are associated with an increased risk of endophthalmitis (vitreous infection), hemorrhage, an increased frequency of cataracts, increased intraocular pressure (a glaucoma risk factor), as well as a risk of retinal tears. Vitrectomy is a major surgical operation and is thus associated with significant risks as well, including vitreous hemorrhage, retinal tears, endophthalmitis, increased intraocular pressure, and a greater risk of cataract formation.[15]
Postoperative and Rehabilitation Care
Despite vitrectomy being a significant surgical operation, vitrectomy operations are generally outpatient procedures that rarely involve general anesthesia.[16]
Deterrence and Patient Education
Patient education is critical; diabetic macular edema is an advanced sequela of diabetes. Patient education can help to prevent, delay, or limit the effects of diabetes. Patients need to understand that diabetes is a systemic disease that can affect organ systems that they were likely unaware that it could affect, including the eyes.
Enhancing Healthcare Team Outcomes
Diabetes is a complex disease that has systemic manifestations best managed by an interprofessional team. Patient education is critical. This starts at the primary care level with primary care providers describing the consequences of diabetes and poor diabetes control. Diabetes and ophthalmology nurses can play a key role in this role as patient educators. The nurses arrange for follow up and keep the rest of the team up to date about the status of patients. Pharmacists can serve as patient advocates with innovations in diabetes management through medication counseling and management, lifestyle counseling, and informing the clinician when appropriate. Patient outcomes are improved when patients understand the full range of manifestations of their underlying pathology. [Level 5]