Continuing Education Activity
Homonymous hemianopsia (HH), or hemianopia, is a visual field deficit affecting corresponding halves of both eyes, often resulting from cerebrovascular injury or tumor. This activity reviews the characteristic features, etiology, and diagnostic techniques for HH, emphasizing the differentiation from other visual field defects. It explores the significance of localized cerebrovascular insults or tumors and their implications in HH onset, guiding healthcare professionals in effective localization and management strategies. Furthermore, the crucial role of an interprofessional team in assessing and addressing the complexities associated with HH is covered, particularly in stroke or pediatric cases. The program aims to enhance participants' proficiency in HH assessment, leading to improved patient care and management.
Objectives:
Identify the various etiologies associated with homonymous hemianopsia (HH), such as cerebrovascular accidents, tumors, traumatic brain injuries, vascular malformations, demyelinating diseases, and posterior cerebral artery infarctions.
Differentiate between HH and other visual field defects, including bitemporal hemianopsia, quadrantanopia, scotoma, or peripheral visual field defects, by understanding their distinctive patterns and associated lesions or pathologies.
Apply compensatory strategies and visual aids suitable for patients with HH, such as prisms, visual scanning training, and exploration therapies to enhance awareness of the visual field loss and improve visual functioning.
Implement multidisciplinary care involving occupational therapists, neurologists, ophthalmologists, and optometrists to develop a comprehensive rehabilitation plan to optimize patients' daily functioning and quality of life.
Introduction
Homonymous hemianopsia (HH), or homonymous hemianopia, is a visual field loss in the same halves of the visual field of each eye. For example, in right HH, the visual field loss is on the right side in the right eye and on the right side in the left eye. This condition most commonly results from a stroke in adults or tumors or lesions in patients under the age of 18.[1] Often, the cause of HH is located at the occipital lobe, followed by an injury to the optic radiations or optic tract.[1] Contrary to HH, bitemporal hemianopsia, also called heteronymous hemianopia (involvement at the optic chiasm) involves visual field loss in the temporal side of either eye.[2]
Etiology
HH frequently results from vascular injury. In adults, cerebral infarcts and intracranial hemorrhages are the most common (42% to 89%) causes.[3] They are followed by tumors, trauma, iatrogenic events, demyelinating disorders, and neurologic disease.[3] Pediatric cases often originate from neoplasms (39%), stroke (25%), and trauma (19%).[4]
Any injury to the retrochiasmal visual pathway may result in HH. Various disorders causing HH include:[5]
- Vascular: Stroke, venous thrombosis, vasculitis, arteriovenous malformation,[6] vertebrobasilar dolichoectasia[7]
- Neurologic: Seizures, posterior cortical atrophy, multiple sclerosis, sarcoidosis, Alzheimer's disease, Creutzfeldt–Jakob disease,[8] corticobasal degeneration (CBD) or corticobasal syndrome (CBS),[3] progressive multifocal leukoencephalopathy
- Infectious: Abscess, encephalomyelitis, toxoplasmosis, cysticercosis, neurosyphilis [1]
- Inflammatory disease: Demyelinating diseases including multiple sclerosis, neuromyelitis optical [9]
- Neoplasm: Metastasis to the brain, lymphoma, other tumors
- Iatrogenic: After neurosurgical procedures, radiation necrosis [10]
- Trauma: Traumatic brain injury, shaken baby syndrome [11] and
- Metabolic diseases: Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes (MELAS) [12]
Causes of transient or temporary HH that resolve spontaneously include:
- Transient ischemic attack
- Migraine
- Occipital, temporal, or parietal lobe seizure
- Nonketotic hyperglycemia [1]
In a large study, the locations of lesions causing HH included:[3]
- Occipital lobe (45%)
- Optic radiation (32.2%)
- Combination of multiple areas (11.4%)
- Optic tract (10.2%)
- Lateral geniculate nucleus (1.3%)
Epidemiology
In a review of 904 cases by Zhang et al, HH appeared in a nearly even distribution among genders (52% male, 48% female) with an average age of around 50 years old.[3] An increased likelihood of left-sided HH (55%) to right-sided HH (45%) was noted. Incomplete HH (HH with visual field loss less than complete HH) was more common (62.4%) than complete HH (ie, HH respecting the vertical meridian involving the entire hemifield on one side with macular splitting) (37.6%), with the most common incomplete presentation being homonymous quadrantanopia (29.2%). HH appeared as an isolated deficit in 46.5% of patients, whereas 53.5% experienced motor symptoms, cognitive changes, or a combination of the two.[3]
Pathophysiology
Structure and Function
The optic nerves run near the midline inferior to the frontal lobe and superior to the cavernous sinus on each side. Their purpose is to transfer retinal information to the appropriate region of the occipital primary visual cortex.[13] The right half of each eye's visual field is recorded by the left half of each retina and vice versa. Ganglion cells within the retina have sensory afferent fibers that course through the optic chiasm. At the chiasm, the nasal portion of each optic nerve crosses to the opposite side, so each eye transmits information through its ipsilateral temporal fibers and the contralateral nasal fibers to the brain.[13]
It has been shown that around 53% of the total optic nerve fibers cross at the optic chiasma, and 47% of the optic nerve fibers do not cross.[14] By crossing at the optic chiasm, all fibers recording the left visual field of both eyes project to the right hemisphere, and the same applies to the right visual field. The optic chiasm is located at the midline inferior to the hypothalamus and superior to the pituitary gland.[13] Once distal to the chiasm, the fibers collectively are named the optic tract. The tract then communicates with the lateral geniculate nucleus (LGN) within the thalamus.
From the thalamus, the fibers enter the optic radiations (the geniculostriate pathway or geniculocalcarine tract) before arriving at the primary visual cortex within the occipital lobe around the calcarine sulcus (or calcarine fissure).[13] The inferior bank of the calcarine fissure (lingual gyrus) and the superior bank of the calcarine fissure (cuneus) form the primary visual cortex (visual area 1 or V1, striate cortex, or Brodmann area 17).[15] Simultaneously, some fibers from the optic tract also communicate with the superior colliculus, pretectal nuclei, and suprachiasmatic nuclei, which are involved in multiple physiologic functions, including the light reflex.[13]
Embryology
Development and differentiation of the eyes begin around week 3 of gestation.[16] The retina and optic nerves are derived from neural ectodermal tissue.[17] As a result, the optic nerve is technically part of the central nervous system. Further supporting this claim is that the myelin sheaths surrounding the optic nerve are arranged by oligodendrocytes as opposed to Schwann cells.[18] At about the seventh week of gestation, axons from the retinal ganglion cells begin to extend into the optic stalk, eventually forming the optic nerve.[16]
Vascular Supply
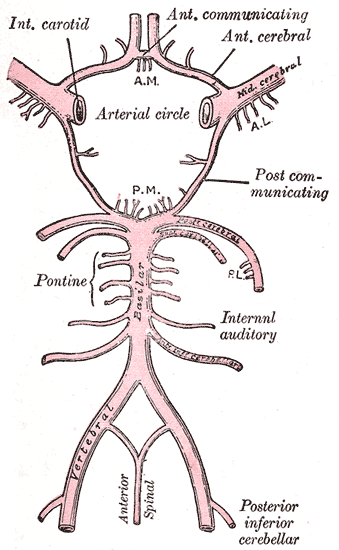
The major vascular supply to the anterior intracranial structures is the circle of Willis (see Image. Circle of Willis) and its associated branches. The optic nerve is supplied by the internal carotid artery (ICA), specifically the superior hypophyseal and ophthalmic arteries.[19] The primary supply to the optic chiasm is delivered by the anterior cerebral (ACA), anterior communicating, posterior communicating, and superior hypophyseal arteries.[20] The ICA and posterior communicating arteries feed the optic tract.[13] The LGN is mainly supplied by the ICA and posterior cerebral artery (PCA).
The optic radiations are divided into anterior and posterior portions, and so are their vascular supplies. Branches from the circle of Willis and the middle cerebral artery (MCA) supply the anterior proximal sections, whereas branches of the PCA supply the posterior distal radiations. A majority of the visual cortex is supplied from branches of the PCA. However, there are also regions of the occipital pole that are additionally supplied by branches of the MCA.[13]
Neuroanatomical Pathway
Each optic nerve transports information from the retinal ganglion cells to the optic chiasm and through the retrochiasmal pathway to varying areas of the brain for use in several ways. Each optic nerve contains around 1.2 million nerve fibers. Nearly 2.4 million fibers exist from the optic nerve that then pass through to the optic chiasm.[21] As mentioned earlier, the temporal fibers communicate visual information to the lateral portions of the optic chiasm, occupying about 35%-45% of the chiasm before continuing to the ipsilateral LGN.[21][22]
As a result, each optic tract leaving the chiasm contains a mix of contralateral nasal (crossed) and ipsilateral temporal (uncrossed) fibers. These fibers receive information from their opposite visual field (eg, the left temporal fibers view the left nasal field). Thus, the left optic tract carries the right half of each eye's visual field and vice versa. Both optic tracts travel near the basal ganglia, internal capsule, and cerebral peduncle on each side.
Most of the nerve fibers from the optic tract enter the ipsilateral LGN. Some of the remaining project to the pretectal nucleus of the same side. Fibers from the pretectal nucleus go to bilateral Edinger-Westphal nuclei (afferent signal for pupillary light reflex).[23] The LGN contains an array of axons distributed in 6 laminae or layers that receive the signal from either the ipsilateral or contralateral eye. Such a retinotopic organization is crucial to properly merge binocular images at the occipital cortex. The contralateral fibers go to laminae 1, 4, and 6. Laminae 2, 3, and 5 receive ipsilateral information.[24]
From the LGN, neurons spread to the primary visual cortex via optic radiations. These optic radiations, also called geniculocalcarine tracts, are divided into superior and inferior segments (sometimes also termed posterior loop and anterior Meyer loop). Like the relationship between nasal and temporal fibers and their visual fields, superior radiation projects inferior field information, and inferior radiation projects superior field information. The superior pathway courses posteriorly through the parietal lobe before contacting the visual cortex. The inferior radiation first courses anteriorly, along the roof of the lateral ventricle's temporal horn, before turning laterally and projecting through the temporal lobe as a Meyer loop.[25] Alternate names for optic radiation traveling through the parietal lobe were introduced as "Baum's loop" or "Shin's loop" by anonymous authors in the Wikipedia article "Optic radiation." [26] Thus, the term Baum's loop is discouraged.[26]
At the primary visual cortex, Brodmann area 17, even further organization of visual information takes place to best merge the images received from each eye. Similar to the rest of the pathway, the visual cortex is also retinotopically arranged. In this way, the central field of vision (macular portion) is received by the posterior pole of the visual cortex.[27] The superior bank of the calcarine sulcus receives visual data from the inferior fields, and the inferior bank is responsible for the superior visual field. The fibers in the primary visual cortex are closely arranged, giving rise to congruous visual field defects, whereas fibers of the retrochiasmal pathway, which are anterior (including optic tract and optic radiation), are not arranged very closely and lead to incongruous visual field defects.
It is believed that more posterior lesions cause more congruous visual field defects. However, a large series of cases with HH found that around 60% of optic radiation lesions and 50% of optic tract lesions cause congruous HH, especially if the lesions are due to a cerebrovascular accident (CVA).[28] Around 17% of patients with occipital lobe lesions may show incongruous visual field defects.[28] Specifically, the rule of congruency may not apply to the lesions affecting the optic tract.[28]
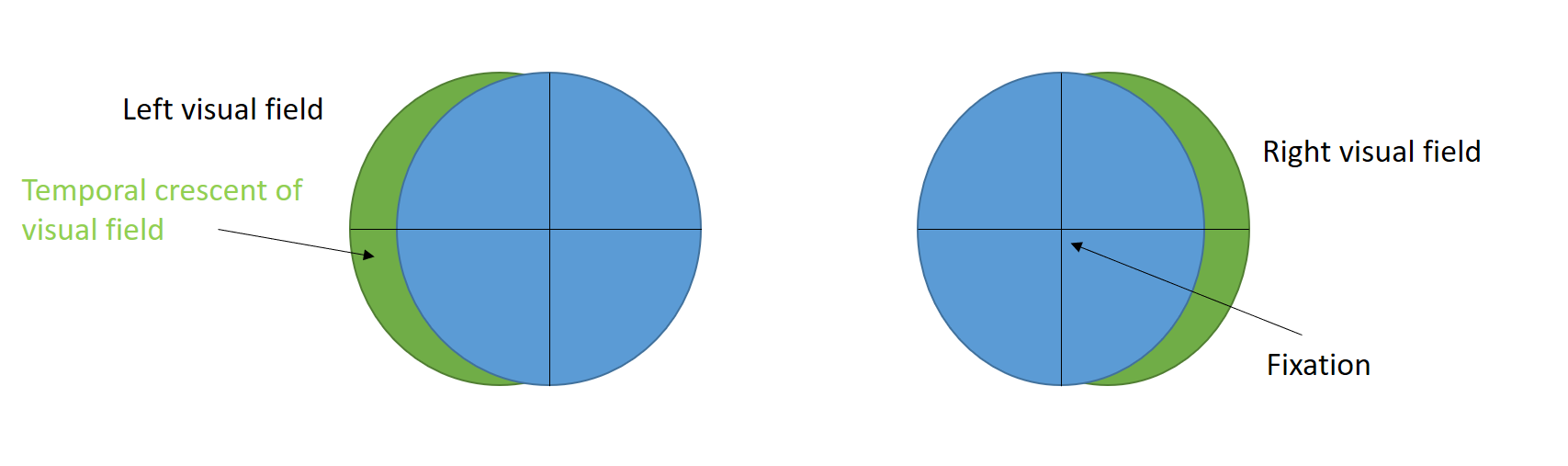
The visual field in each eye extends 60° degrees from the fixation nasally and superiorly (up to 80° inferiorly and 90°-100° temporarily).[29] Thus, the temporal visual field of one side is larger than the nasal visual field of the other eye. This means that the peripheral temporal crescent of the visual field (60° to 90° or 100°) has no perimetric corresponding visual field in the fellow eye (see Image. Temporal crescent). This size discrepancy also correlates with the fact that the number of nasal fibers crossing at the chiasma (serving the temporal visual field) is greater than those serving the nasal field in a 53:47 ratio.
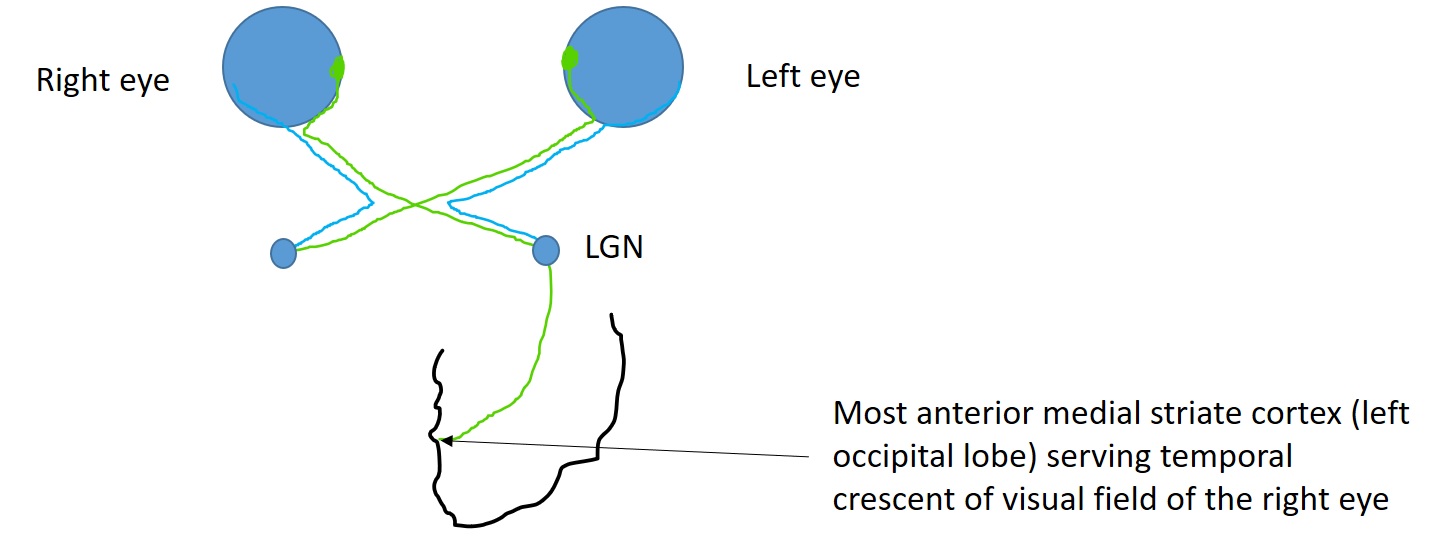
The anterior-most medial striate cortex of the occipital lobe is responsible for processing the temporal crescent of the visual field of the contralateral eye (see Image. Visual pathway schematic). Damage to this area of the occipital lobe does not cause any visual field defects in the ipsilateral eye. Damage to the anterior-most medial striate cortex or anterior part of the parieto-occipital sulcus near the midline results in monocular visual field defect in the contralateral eye (half moon syndrome or temporal crescent syndrome).[30] Such visual field defects lie in the extreme periphery and can not be detected in standard Humphrey 30-2 or 24-2 protocols. Although retrochiasmal lesions usually cause bilateral visual field defects (HH), unilateral visual field defects are possible with anterior occipital lobe lesions.
Corresponding projections to the retinal ganglion cells of the visual fields can be vertically divided into temporal and nasal halves. Concerning HH, the field deficit can be described as complete or partial/incomplete. The involvement of the entire half is complete hemianopsia, whereas any other loss is partial.
Complete Hemianopsia
Complete HH is a visual field deficit with loss of the complete hemifield on the affected side bilaterally, including that half of the macula. Lesions at any point of the retrochiasmal visual pathway can cause this defect.[3]
Partial or Incomplete Hemianopsia
Homonymous quadrantanopia
Often partial but sometimes complete, homonymous quadrantanopia is a visual field deficit with a superior or inferior component in addition to the nasal or temporal involvement. This presents itself as a loss of one quadrant of the visual field. Most often, damage to the superior or inferior banks of the primary visual cortex (eg, infarct) will present as a bilateral quadrantanopia. Quadrantanopia may also result from unilateral superior or inferior optic radiation involvement. For instance, right-sided Meyer loop (inferior radiation) damage would cause vision loss in the superior-left quadrant bilaterally (pie in the sky).[31][32] The damage to the right optic radiation passing through the parietal lobe causes vision loss in the inferior left quadrant bilaterally (pie on the floor defect).
HH with macular sparing
Sparing of the macula permits a centrally localized (about 5°-25°) region of functional vision in the side affected with HH, leading to an incomplete HH. Commonly, this deficit results from infarcts or strokes involving the most posterior portion of the occipital cortex in a classic posterior cerebral artery distribution. The theories to explain this include:
- The macular representation at the occipital lobe is disproportionately large. Around 50%-60% of the visual cortex serves the central 10°-30° field of vision.[33][34]
- The occipital pole, which manages macular field information, has an anastomotic vascular supply with the contralateral side. This tends to abate macular field loss while the surrounding tissue and the surrounding visual field remain involved.[3]
- The macular area is supplied by both the posterior cerebral artery and the middle cerebral artery. In strokes involving the posterior cerebral artery, the macular area is still perfused by the middle cerebral artery.[35]
- The macula on one side is represented in both occipital lobes so that if the occipital lobe of one side is affected, the backup representation from the occipital lobe of the other side remains.[35]
However, macular sparing may also be seen in lesions involving optic radiation or the optic tract.[3]
Homonymous scotomatous defects
Homonymous scotomatous defects are rare visual field losses limited to the central 30°, respecting the vertical meridian, and surrounded by functional peripheral vision. These deficits are most often caused by occipital tip injury (infarct or stroke) that damages the cortex beyond the dual vascular reserve. As a result, this field loss can be described as a reversal from macular sparing hemianopsia.[3]
Homonymous sectoranopia
Homonymous sectoranopia is a rare defect resulting in wedge-shaped field loss. The loss is often a result of the involvement of the lateral geniculate ganglion, which has a dual vascular supply from both anterior and posterior choroidal arteries. Anterior choroidal artery involvement characteristically causes a superior and inferior wedge-shaped loss straddling the horizontal meridian (see Image. Anterior choroidal artery infarct). Posterior choroidal artery involvement typically spares the superior and inferior sectors and has a field loss of the wedge in between (see Image. Posterior choroidal artery infarct).[36][37]
Temporal crescent–sparing
Temporal crescent–sparing, or unilateral loss, involves varying field loss of about 30° of the farthest peripheral temporal field (60°-90°), which is not overlapped by the contralateral eye's nasal field (the temporal crescent), as already discussed. This temporal crescent defect is noted in the eye contralateral to an anterior occipital lesion.[38]
History and Physical
HH involves loss of visual field zones, and patients often present with bilateral field loss, though sometimes they complain of monocular loss or dyslexia. All the patients complaining of HH should receive comprehensive medical eye evaluation, including visual acuity, pupillary reaction, intraocular pressure, slit-lamp examination, and fundus examination.[39] Confrontation perimetry is a useful bedside method to detect HH. Neurological examination for localizing signs is crucial for clinical suspicion of the location of the lesion. Most commonly, the patients present with symptoms and signs of CVA, including sudden weakness or numbness of the unilateral face, arm, or leg, dizziness, sudden confusion, difficulty in speech or understanding, and loss of consciousness.
There is usually reading difficulty. For people reading from left to right, right HH causes difficulty finding the next word and left HH causes difficulty finding the next line. Usually, right HH is more challenging in this regard.[1] Disorganized ocular movement patterns, prolonged fixation, increased regressive saccades, and decreased amplitude of saccade to the right further increase the difficulty in reading in patients with right HH.[40] As the parafoveal area is crucial for initiating the saccade, patients with macular sparing (3°-5°) may have less difficulty reading.[41]
Patients with right HH have difficulty driving and locating vehicles that pass them in countries where the driver seat is on the right side of the vehicle, including the United Kingdom and India. Similarly, patients with left HH have difficulty driving when the driver's seat is placed at the left of the vehicle (as in the United States). It is known that hemianopic drivers have difficulty detecting pedestrians at the side of their hemianopia and taking appropriate action.[42] Such patients may not meet the binocular visual field criteria for driving and may be legally not eligible to drive in some countries and states.
In patients with hemianopia, driving was deemed safe or satisfactory in around 82% (margin of anticipation), 68% (interaction with other road users), 59% (smoothness or vehicle control skills), 64% (adjustment to traffic speed conditions), and 73% (reaction to unexpected events).[6] Only 27% of patients showed "unusually bad driving maneuvers" in a study evaluating 22 patients with hemianopia.[6] The inability to drive may increase the chances of unemployment, increase dependency, and may lead to depression.
Patients with HH may have difficulty walking across a road, staying in a lane while driving, and responding to the surrounding environment. They usually have more saccades or head turns to the hemianopic side, but the movements are usually not systematic, which increases the search time and time taken to respond to the changing environment.
Optic Tract Lesions
Optic tract lesions may result in complete or incomplete hemianopsias. The involvement of the optic tract often includes afferent pupillary nerve fibers. This can present as a pupillary defect in the eye contralateral to the tract lesion. Other times, the patient may present with a Wernicke pupil (ie, light shone on the functioning half of the affected eye will cause pupillary constriction). However, light shone to the half with field loss may cause lessened or loss of pupillary constriction.
On retinal exams, often characteristic optic atrophy can be visualized. The optic disc of the eye contralateral to the lesion shows a horizontal "bow-tie" about one to one-and-a-half months after injury. The ipsilateral eye may present with diffuse optic disc pallor. Less common are signs of damage to surrounding anatomical structures, such as the hypothalamus and internal capsule. Visual acuity and color perception are often preserved unless there exists bilateral involvement of the anterior extension of the lesion involving the optic nerve or chiasm.[43]
LGN
The LGN has a complex array of neurons and axons correlated with the retinotopic arrangement of the eye. Because of this, defects tend to be variable and incomplete. As stated above, characteristic wedge-shaped field losses (sectoranopia) are seen depending on the involvement of the anterior or posterior choroidal arteries. Unless further involvement of the optic tract occurs, patients present with normal pupillary reflexes. Signs of ipsilateral thalamic or pyramidal tract involvement may also be present.[36][37]
Optic Radiations
The involvement of the optic radiations often causes complete, contralateral homonymous hemianopsia. Injury to the radiation can occur proximally or distally. Because of the anatomical relationship with the posterior limb of the internal capsule near the proximal radiations, corticospinal and thalamocortical nerve fibers are often involved with lesions in this location. Distal injury can involve the temporal or parietal lobes. Defects from injury of the optic radiations in the temporal lobe are more likely to be incomplete, superiorly situated "pie-in-the-sky" field losses. Temporal lobe association may also result in aphasia, seizures, hallucinations, or memory complications (when on the dominant hemisphere).
Parietal lobe optic radiations are more often associated with incomplete, inferiorly located lesions. When affecting the nondominant hemisphere, these lesions may produce contralateral hemineglect, differing from the dominant hemisphere (Gerstmann syndrome [acalculia, agraphia, finger agnosia, and right-left disorientation]). The involvement of the parietal lobe may also cause difficulty with smooth pursuit towards the side of the lesion, presenting as nystagmus in that direction.[25][44]
Occipital Lobe
The most common HH deficits are from occipital lobe lesions. These deficits typically present without other associated neurologic symptoms. However, the patterns may vary. Often, these lesions include macular sparing due to the dual blood supply and bilateral macular representation at the occipital cortex. At the occipital pole, most posteriorly, homonymous scotomas are produced. Most anteriorly, temporal crescent loss and other similar peripheral vision patterns occur. If the lesion extends anteriorly enough to involve the left corpus callosum, patients may present with alexia without agraphia (if the lesion spares the angular gyrus).
Bilateral occipital lobe lesions will produce bilateral HH of various types. Most notable is Anton's syndrome, involving a complete bilateral homonymous hemianopsia (cortical blindness), with which the patient also experiences anosognosia (being unaware of their blindness).[45] With bilateral occipital lesions, it is possible that visual acuity may be impacted.[1][33][46]
Evaluation
The visual field confrontation test is the most commonly used screening tool to assess for visual field defects. Typically, the patient will cover one eye and be asked to look at a stationary object (usually the eye of the examiner) while noting the number of fingers visible in their periphery. This test, however, is instructor-dependent and has low sensitivity. Another option is the automated or manual (Goldmann, tangent screen, Humphrey visual field) perimetry test.[29] This test provides information not only on the field loss but also on the size and form of the deficit.[47] The depth of deficit is also known by using a perimeter.[48]
Visual field defects due to parietal lobe lesions are denser or deeper inferiorly. Goldmann's visual field is used for neurological visual field defects. However, it may not be available in all clinical settings. Humphrey Matrix frequency-doubling technology (FDT) perimetry (24-2) did not show statistically significant differences compared with Humphrey Visual Field Analyzer (Swedish Interactive Threshold Algorithm [SITA] standard program 24-2, standard automated perimetry [SAP]) in the detection of HH in a study evaluating 33 patients with HH.[49] However, the sensitivity of SAP may be more than that of FDT, likely due to scattered abnormal locations and the failure of FDT to detect test locations at the vertical meridian.[49][50] A confrontation visual field is not a sensitive method to detect HH, though using a red target may improve the sensitivity.[51]
In addition to identifying the field deficit, all patients experiencing HH should undergo further neuroimaging, such as an MRI (magnetic resonance imaging) or computed tomography (CT) of the brain, to identify the cause of such symptomatology.
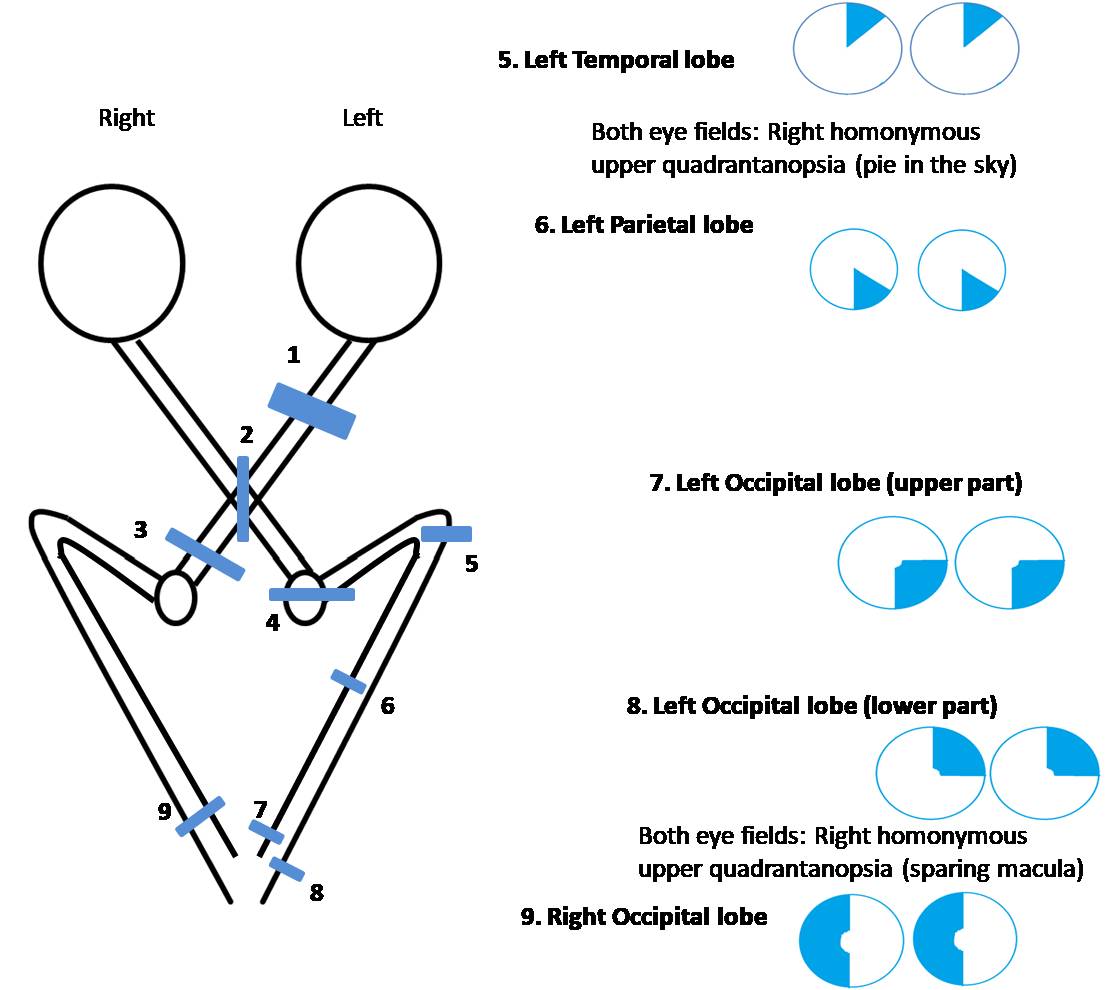
Localization of Lesions
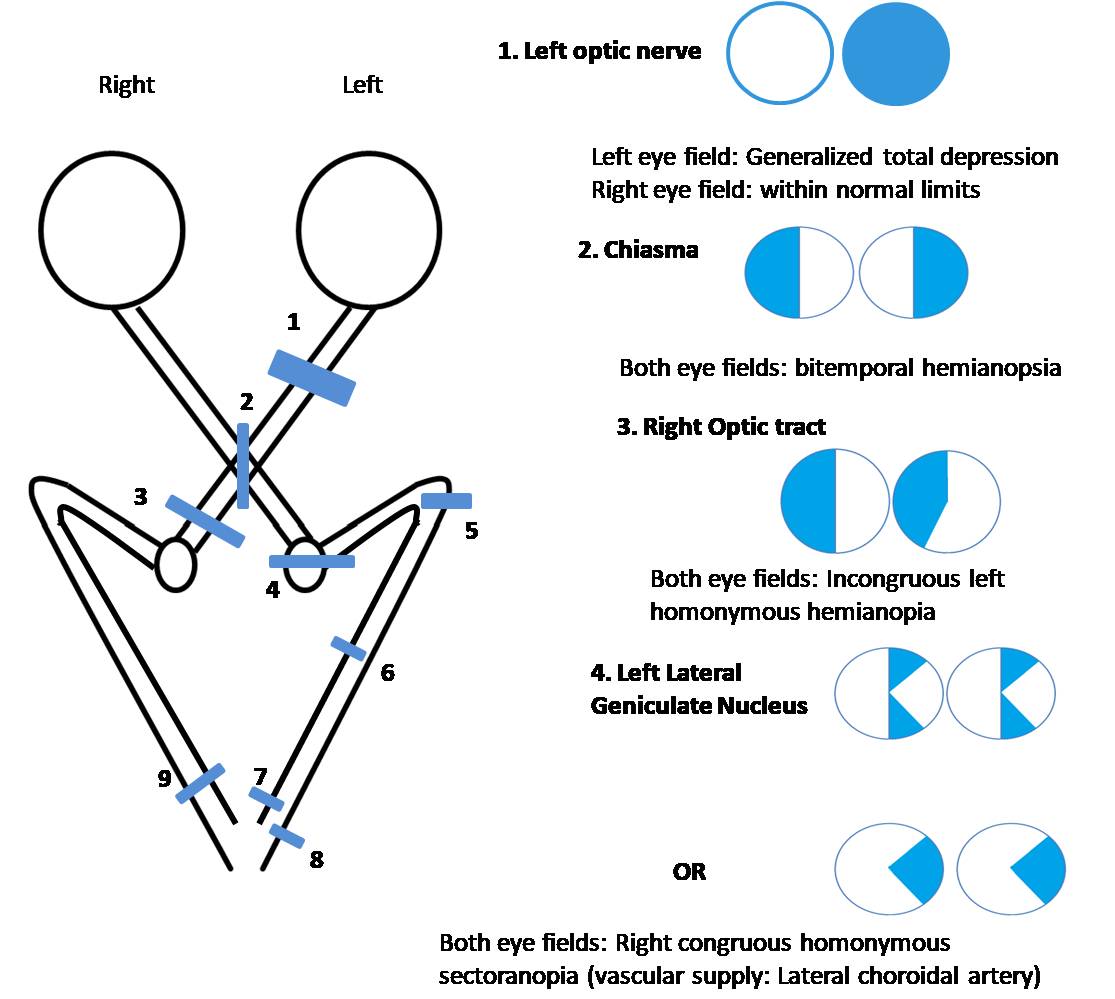
A relative afferent pupillary defect (RAPD) or Marcus Gunn pupil in a patient with HH denotes that the lesion is anterior to the lateral geniculate nucleus involving the optic tract. Lesions posterior to the LGN do not cause RAPD. Another important sign is the similarity of the visual field defects between the right and left eyes. If the visual field defect is identical or very similar in each eye (congruous), the lesion is likely to be posterior (including the occipital lobe), though clinical variations are not uncommon.[28] If the visual field defect in the right and left eye is not similar, it is called an incongruous visual field defect, and the lesion is usually anterior.
Although variations may exist, common clinical scenarios include:[50]
- Left optic tract lesion: HH on the right side (in each eye) with a relative afferent pupillary defect in the right eye and band atrophy of the right optic nerve [52]
- Left temporal lobe (Meyer's loop): HH on the right side (in either eye) that is denser superiorly (pie in the sky) with incongruous visual field defect, no RAPD
- Left parietal lobe: HH on the right side (in either eye) that is denser inferiorly (pie on the floor) with incongruous visual field defect, no RAPD
- Left occipital lobe: HH on the right side (in either eye) with congruous visual field defect, no RAPD, macular sparing
- Anterior medial striate cortex of the left occipital lobe: Loss of extreme temporal field of vision in the right eye with normal left visual field
Treatment / Management
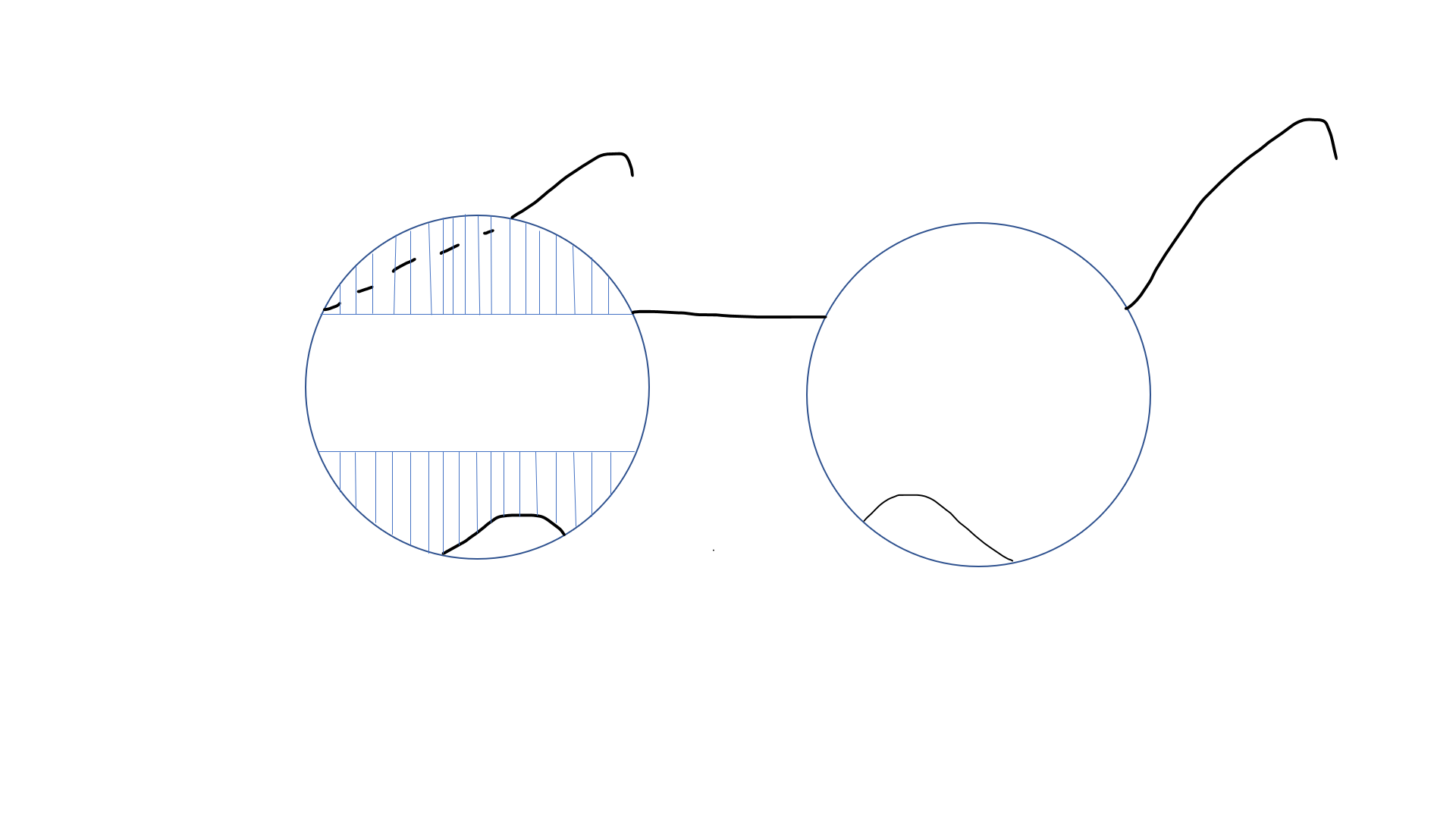
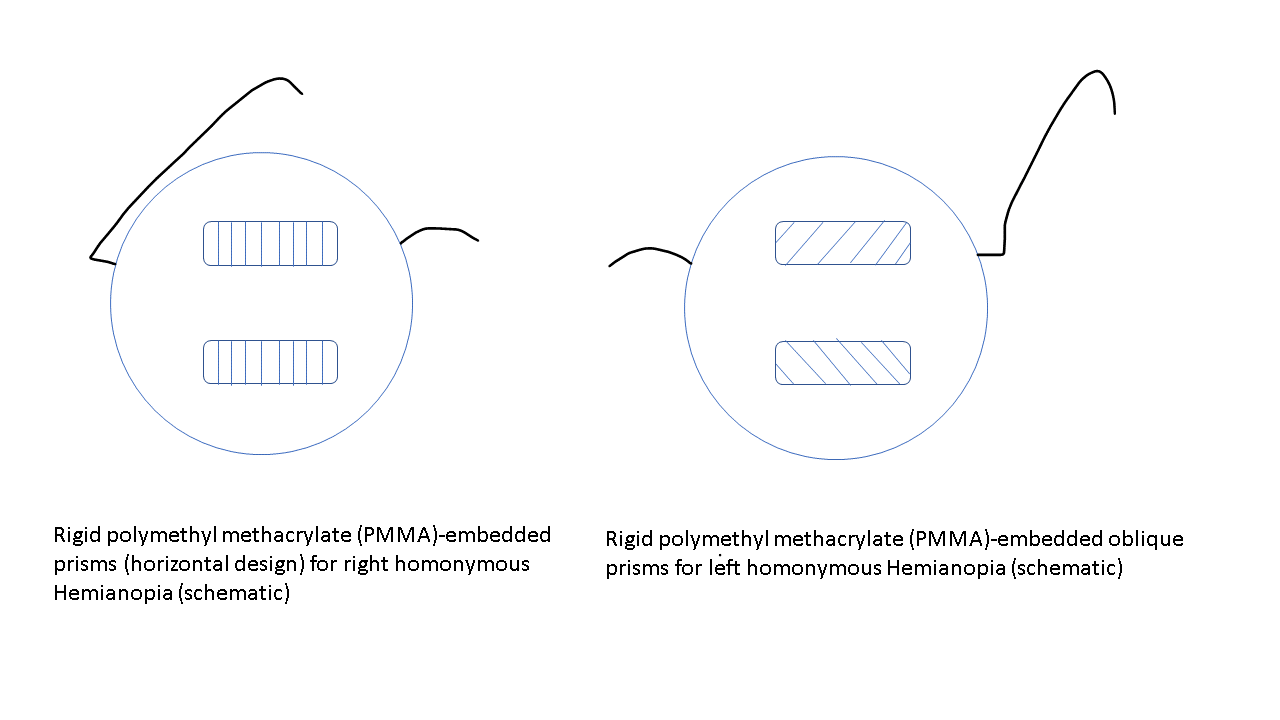
The treatment of patients with HH needs a multidisciplinary approach. The visual deficit on the hemianopic side can be reduced with 2 prisms that are placed in the upper and lower part of the spectacle (see Image. Peli prisms).[53] These prisms are only placed on one eye (on the side of HH) (eg, for the right HH, these prisms are worn over the right eye). The base of the prism is toward the hemianopic side (base out). This gives the view of objects lying on the hemianopic side and expands the intact visual field while avoiding diplopia, which may occur if prisms are placed in the line of sight. These prisms may include a single-mounted prism or compound Fresnel press-on prism. The expansion of the field may be quite satisfying (up to 20° with 40 prism diopter segments and 30° with 57 prism diopter segments).[54][55] Variants include short segments of prism just above and below the line of sight (Peli lens, rigid polymethyl methacrylate [PMMA]-embedded prisms (see Image. PMMA-embedded prisms).[56]
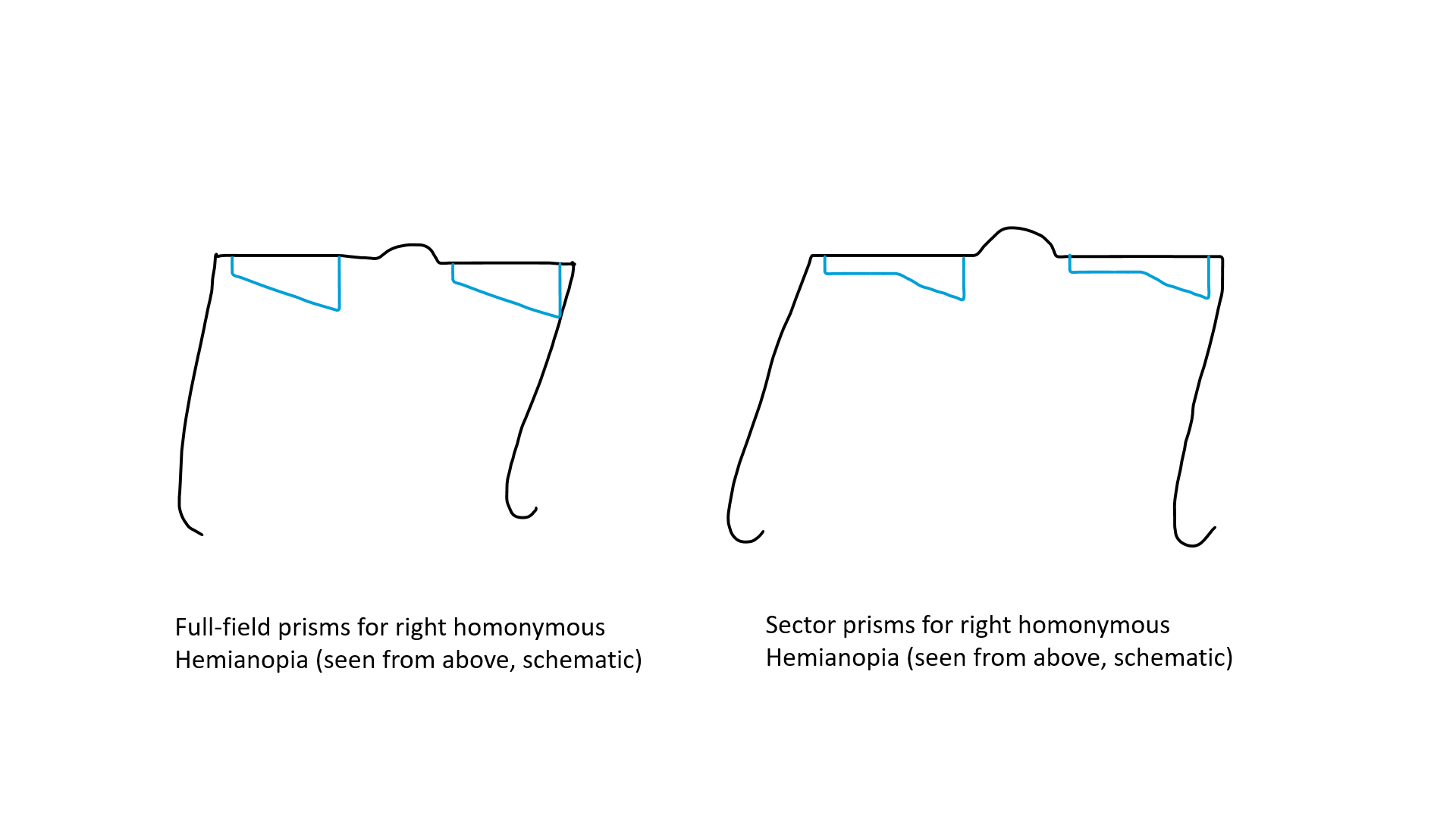
Previously, full-field yoked prisms filling the entire glass of spectacle of either eye (with base towards the blind side) had been used, which reduced visual acuity and contrast sensitivity and did not increase the field of vision (see Image. Yolked prisms). Bilateral sectoral or partial prisms that lie on the blind side of each eye have also been used. The sector prisms cause apical scotoma and may cause practical difficulty during the movement of the patient.[56] Multiperiscopic prism device is another approach to expanding the visual field.[57] Obliquely oriented prisms may expand the central visual field while avoiding diplopia.[58] The patients need training for using the prism. Once they detect that something is approaching from the blind side, they should move their head to see the area clearly. Challenges with prisms include reduced optical clarity, disorientation, difficulty in using stairs, and inability to read with prisms. Compensatory training for planned, systematic eye and head movements and systematic scanning tactics may improve mobility and result in efficient and reduced search time.[59] Visual training, computer-based therapy, and vision restorative training are other approaches to improving the visual functions of patients with HH.
Occupational therapy, social support, and psychological rehabilitation are crucial in the management of patients with HH. Other therapies include working with visual field loss. For instance, programs that teach patients how to read with assistance (following the text with a finger) or move their eyes into the field loss (saccadic training) may improve overall functionality.[60][61]
Differential Diagnosis
A differential diagnosis of visual field loss is glaucoma. Compared with neurological field loss (like HH), which obeys the vertical meridian, the visual field loss due to glaucoma characteristically obeys the horizontal meridian.[62] Homonymous hemianopia causes visual field loss on the same side in either eye (in the right homonymous hemianopia, there is field loss on the right side in both the right eye and left eye). In heteronymous hemianopia, the side of visual field loss is different in each eye (like in bitemporal hemianopia, the right-sided visual field is lost in the right eye and left-sided visual loss is there in the left eye).
Prognosis
Spontaneous improvement of HH varies dramatically, with great emphasis on the timeline from when the injury occurred to when the visual field test was administered. Around 20% to 50%-60% of patients with HH (after cerebral stroke) may have spontaneous improvement of HH within 1 month.[63][64][65] The improvement occurs in most cases within 3 months; spontaneous improvement after 6 months is rare. It may be related to improving underlying disease or patients' ability to perform perimetry reliably.[65] In a study evaluating 254 patients with HH, the chance of improvement of the visual field was 50% to 60% in patients tested within 1 month after injury compared with 20% in patients tested 6 months after surgery.[65] Improvement in underlying diseases has been shown to provide visual field improvement even after 6 months (eg, multiple sclerosis).[64][65]
Complications
In addition to the visual field deficit, patients experiencing HH may feel disoriented and complain of dizziness, vertigo, or nausea. These symptoms all increase the risk of trauma. Patients are more likely to fall as a result of their field loss.
Deterrence and Patient Education
Risk factors for HH are those related to the common causes. Vascular insult being most frequent, patients should be aware of their possible stroke risk. Atherosclerosis, hypertension, diabetes, smoking history, obesity, heavy alcohol intake, and previous vascular injury are all factors.[66] For patients already experiencing HH, driving is a serious concern. Patients should know that operating machinery with a visual field loss may put themselves and others at risk.[67] Each state has different visual criteria regarding driving licensure. If the state determines that the patient does not meet the minimum visual criteria, the physician should counsel the patient on driving and safety.
Enhancing Healthcare Team Outcomes
HH is a field loss deficit in the same halves of the visual field of each eye. Patients with HH may also exhibit nonspecific signs and symptoms such as nausea, coordination difficulty, vertigo, and dyslexia. HH is often due to a myriad of diagnoses, including metabolic, neurologic, immunologic, traumatic, infectious, and vascular etiologies. Although a physical exam may reveal a visual field deficit, the cause is difficult to discern without proper imaging studies.
A primary care or emergency physician is almost always involved in caring for patients with a visual field deficit; however, it is important to consult with an interprofessional team of specialists, including an ophthalmologist, neurologist, neurosurgeon, radiologist, or interventional radiologist. Nurses are also vital members of the interprofessional group, as they will monitor the patient's vital signs and assist with educating the patient and their family. If the causal lesion is classified as surgical in nature, patients may undergo an operation as part of the treatment plan. In the postoperative period, the pharmacist will ensure patients are on the proper analgesics, antiemetics, and appropriate antibiotics for pain, wound infection, and ileus.
The radiologist also plays a vital role in determining the cause of HH. Although it is most commonly secondary to cerebrovascular injury, trauma, or tumors, the specific location of the causal lesion can be difficult to glean from the visual field loss alone. Prompt consultation with an interprofessional group of specialists is recommended to best improve outcomes.[3] In addition, patient adaptation after experiencing visual field loss continues to be explored. Therapy options vary and are best followed through with the inclusion of multiple specialists.[68]

![The images shows visual field defect due to anterior choroidal artery infarct [horizontal sector sparing homonymous hemianopia] affecting lateral geniculate body](/pictures/getimagecontent//19904)