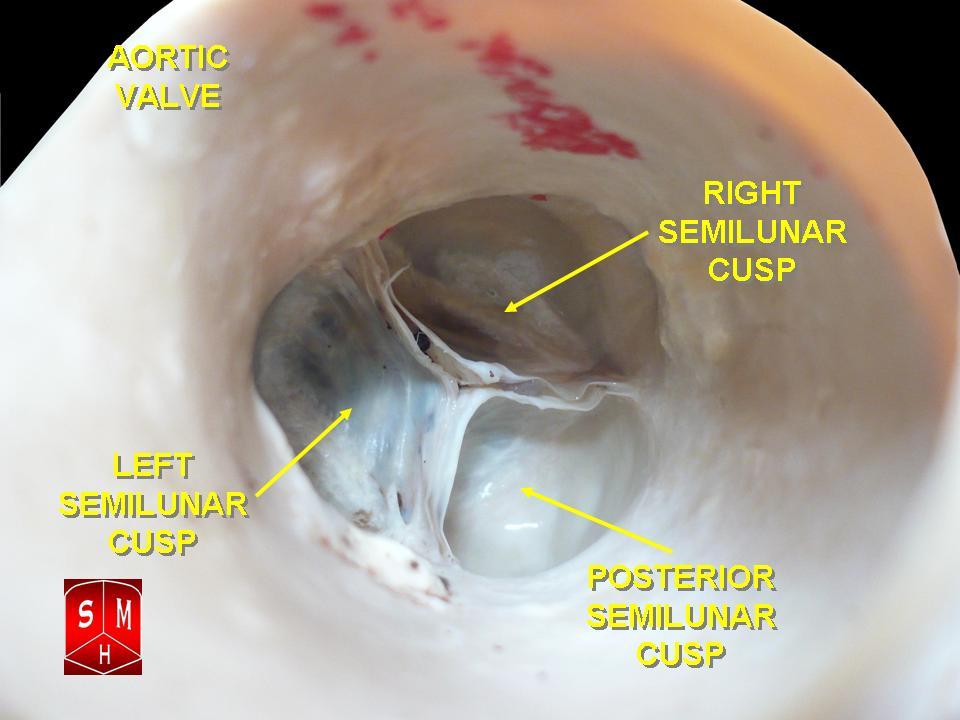
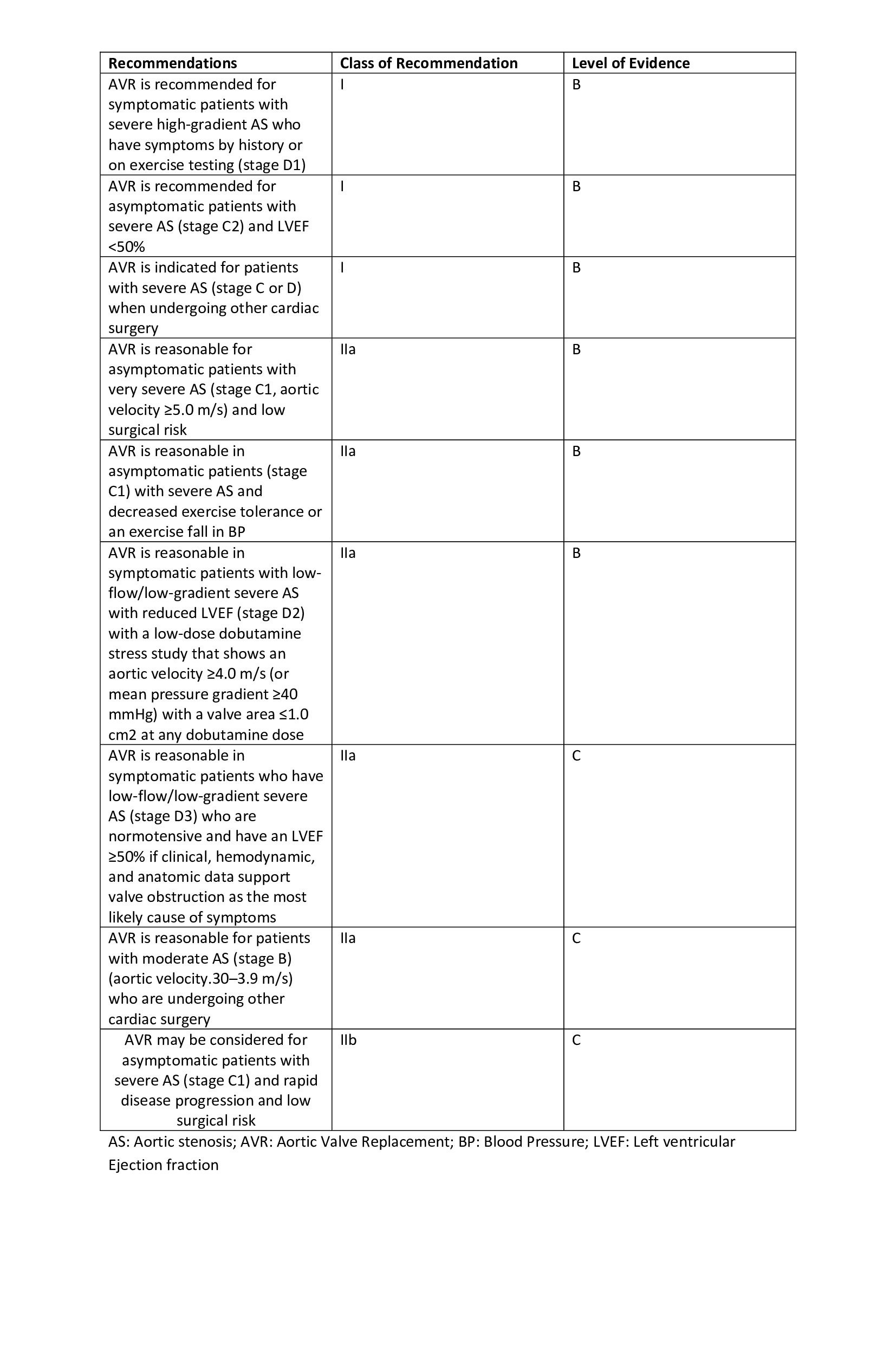
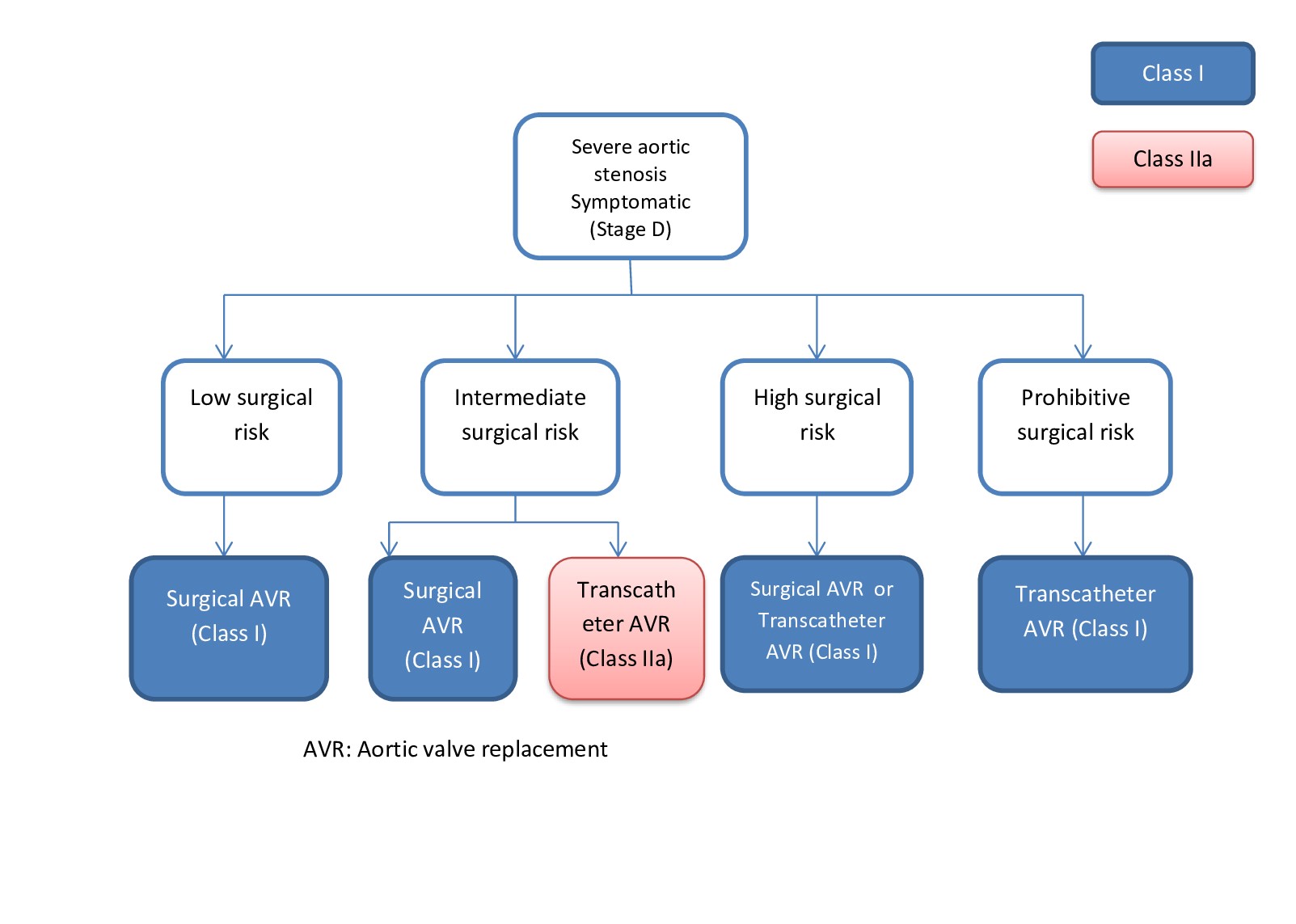
[1]
Cribier A, Eltchaninoff H, Bash A, Borenstein N, Tron C, Bauer F, Derumeaux G, Anselme F, Laborde F, Leon MB. Percutaneous transcatheter implantation of an aortic valve prosthesis for calcific aortic stenosis: first human case description. Circulation. 2002 Dec 10:106(24):3006-8
[PubMed PMID: 12473543]
Level 3 (low-level) evidence
[2]
Sakata Y, Syed Z, Salinger MH, Feldman T. Percutaneous balloon aortic valvuloplasty: antegrade transseptal vs. conventional retrograde transarterial approach. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography & Interventions. 2005 Mar:64(3):314-21
[PubMed PMID: 15736255]
[3]
Abdelghani M, Cavalcante R, Miyazaki Y, de Winter RJ, Tijssen JG, Sarmento-Leite R, Mangione JA, Abizaid A, Lemos PA, Serruys PW, de Brito FS Jr. Transcatheter aortic valve implantation for mixed versus pure stenotic aortic valve disease. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2017 Nov 20:13(10):1157-1165. doi: 10.4244/EIJ-D-17-00328. Epub
[PubMed PMID: 28691910]
[4]
Barker CM,Reardon MJ, Should TAVR Replace Surgery for Aortic Stenosis in Low- to Intermediate-Risk Patients? The Canadian journal of cardiology. 2017 Sep;
[PubMed PMID: 28843323]
[5]
Arai T, Lefèvre T. Who is the right patient for TAVI? Journal of cardiology. 2014 Mar:63(3):178-81. doi: 10.1016/j.jjcc.2013.11.005. Epub 2013 Dec 12
[PubMed PMID: 24332753]
[6]
Rezq A, Godino C, Montorfano M, Covello D, Colombo A. Comprehensive multidisciplinary patient assessment and selection before TAVI procedure. Minerva cardioangiologica. 2014 Apr:62(2):177-91
[PubMed PMID: 24686996]
[7]
Olasińska-Wiśniewska A, Trojnarska O, Grygier M, Lesiak M, Grajek S. Percutaneous balloon aortic valvuloplasty in different age groups. Postepy w kardiologii interwencyjnej = Advances in interventional cardiology. 2013:9(1):61-7. doi: 10.5114/pwki.2013.34029. Epub 2013 Mar 21
[PubMed PMID: 24570692]
Level 3 (low-level) evidence
[8]
Patel PA, Fassl J, Thompson A, Augoustides JG. Transcatheter aortic valve replacement--part 3: the central role of perioperative transesophageal echocardiography. Journal of cardiothoracic and vascular anesthesia. 2012 Aug:26(4):698-710. doi: 10.1053/j.jvca.2012.03.017. Epub 2012 May 11
[PubMed PMID: 22578977]
[9]
Grube E, Schuler G, Buellesfeld L, Gerckens U, Linke A, Wenaweser P, Sauren B, Mohr FW, Walther T, Zickmann B, Iversen S, Felderhoff T, Cartier R, Bonan R. Percutaneous aortic valve replacement for severe aortic stenosis in high-risk patients using the second- and current third-generation self-expanding CoreValve prosthesis: device success and 30-day clinical outcome. Journal of the American College of Cardiology. 2007 Jul 3:50(1):69-76
[PubMed PMID: 17601548]
Level 2 (mid-level) evidence
[10]
Dapunt OE, Luha O, Ebner A, Sonecki P, Spadaccio C, Sutherland FW. New Less Invasive Approach for Direct Aortic Transcatheter Aortic Valve Replacement Using Novel CoreVista Transcervical Access System. JACC. Cardiovascular interventions. 2016 Apr 11:9(7):750-3. doi: 10.1016/j.jcin.2016.01.035. Epub
[PubMed PMID: 27056316]
[11]
Walther T, Dewey T, Borger MA, Kempfert J, Linke A, Becht R, Falk V, Schuler G, Mohr FW, Mack M. Transapical aortic valve implantation: step by step. The Annals of thoracic surgery. 2009 Jan:87(1):276-83. doi: 10.1016/j.athoracsur.2008.08.017. Epub
[PubMed PMID: 19101311]
[12]
Kogoj P, Devjak R, Bunc M. Balloon aortic valvuloplasty (BAV) as a bridge to aortic valve replacement in cancer patients who require urgent non-cardiac surgery. Radiology and oncology. 2014 Mar:48(1):62-6. doi: 10.2478/raon-2013-0078. Epub 2014 Jan 22
[PubMed PMID: 24587781]
[13]
Rodés-Cabau J, Sacco RL. Neurological Complications Following Aortic Valve Replacement: TAVR Better Than SAVR, But Room for Improvement. Journal of the American College of Cardiology. 2018 Oct 30:72(18):2120-2122. doi: 10.1016/j.jacc.2018.06.080. Epub
[PubMed PMID: 30360821]
[14]
Smith CR,Leon MB,Mack MJ,Miller DC,Moses JW,Svensson LG,Tuzcu EM,Webb JG,Fontana GP,Makkar RR,Williams M,Dewey T,Kapadia S,Babaliaros V,Thourani VH,Corso P,Pichard AD,Bavaria JE,Herrmann HC,Akin JJ,Anderson WN,Wang D,Pocock SJ, Transcatheter versus surgical aortic-valve replacement in high-risk patients. The New England journal of medicine. 2011 Jun 9;
[PubMed PMID: 21639811]
[15]
Ribeiro HB, Webb JG, Makkar RR, Cohen MG, Kapadia SR, Kodali S, Tamburino C, Barbanti M, Chakravarty T, Jilaihawi H, Paradis JM, de Brito FS Jr, Cánovas SJ, Cheema AN, de Jaegere PP, del Valle R, Chiam PT, Moreno R, Pradas G, Ruel M, Salgado-Fernández J, Sarmento-Leite R, Toeg HD, Velianou JL, Zajarias A, Babaliaros V, Cura F, Dager AE, Manoharan G, Lerakis S, Pichard AD, Radhakrishnan S, Perin MA, Dumont E, Larose E, Pasian SG, Nombela-Franco L, Urena M, Tuzcu EM, Leon MB, Amat-Santos IJ, Leipsic J, Rodés-Cabau J. Predictive factors, management, and clinical outcomes of coronary obstruction following transcatheter aortic valve implantation: insights from a large multicenter registry. Journal of the American College of Cardiology. 2013 Oct 22:62(17):1552-62. doi: 10.1016/j.jacc.2013.07.040. Epub 2013 Aug 14
[PubMed PMID: 23954337]
Level 2 (mid-level) evidence
[16]
Beohar N, Kirtane AJ, Blackstone E, Waksman R, Holmes D Jr, Minha S, Alli O, Suri RM, Svensson LG, Leon M, Kodali S. Trends in Complications and Outcomes of Patients Undergoing Transfemoral Transcatheter Aortic Valve Replacement: Experience From the PARTNER Continued Access Registry. JACC. Cardiovascular interventions. 2016 Feb 22:9(4):355-363. doi: 10.1016/j.jcin.2015.10.050. Epub 2016 Jan 20
[PubMed PMID: 26803420]
[17]
Ben-Dor I, Pichard AD, Satler LF, Goldstein SA, Syed AI, Gaglia MA Jr, Weissman G, Maluenda G, Gonzalez MA, Wakabayashi K, Collins SD, Torguson R, Okubagzi P, Xue Z, Kent KM, Lindsay J, Waksman R. Complications and outcome of balloon aortic valvuloplasty in high-risk or inoperable patients. JACC. Cardiovascular interventions. 2010 Nov:3(11):1150-6. doi: 10.1016/j.jcin.2010.08.014. Epub
[PubMed PMID: 21087751]