Continuing Education Activity
Coronary artery disease presents a challenge in diagnosis due to its characteristic chest pain. Despite medical advancements, it remains a significant cause of mortality and morbidity worldwide. To tackle this issue, healthcare professionals require diagnostic methods that are both timely and cost-effective. One such method is coronary computed tomography angiography, which is recommended for patients with an intermediate probability of coronary artery disease. This non-invasive assessment is crucial in helping clinicians diagnose and manage chest pain. This activity provides an overview of the indications, contraindications, and clinical relevance of coronary computed tomography angiography. It also highlights the importance of an interprofessional team approach when managing patients with chest pain.
Objectives:
Identify appropriate candidates for coronary computed tomography angiography based on clinical indications and pre-test coronary artery disease probability.
Differentiate between the indications and contraindications for coronary computed tomography angiography compared to other coronary artery disease evaluation modalities, such as stress testing or invasive coronary angiography.
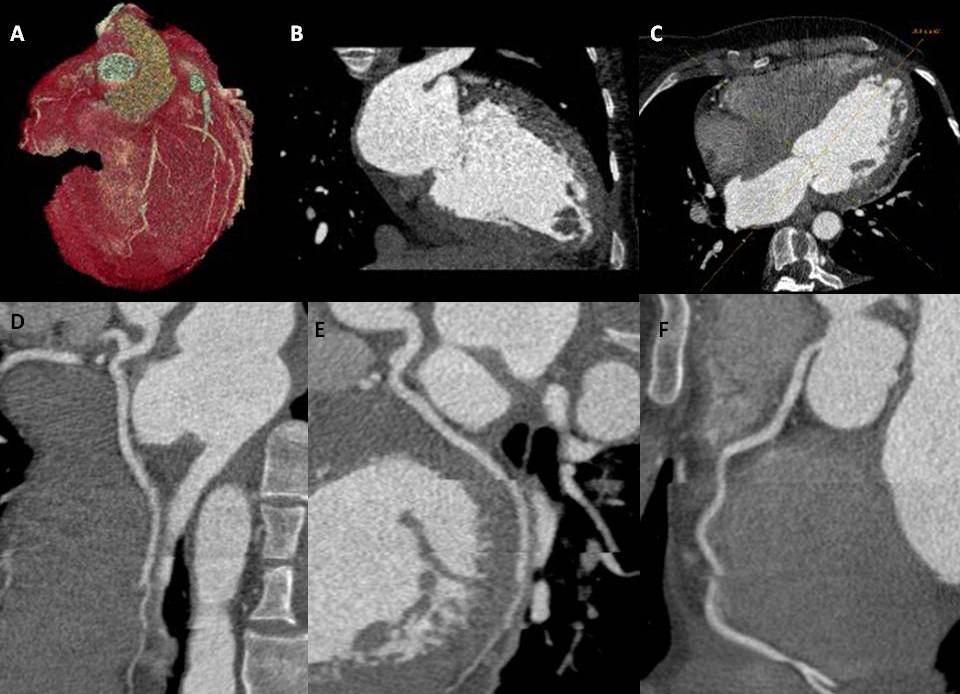
Assess coronary computed tomography angiography images effectively to identify and evaluate coronary artery stenosis, plaque characteristics, and other relevant findings.
Collaborate with radiologists and cardiologists in interpreting coronary computed tomography angiography results and developing appropriate treatment plans.
Introduction
Chest pain is the most common symptom of coronary artery disease (CAD), posing a significant diagnostic challenge for clinicians. Despite remarkable strides in medical and procedural treatments, cardiovascular disease persists as a major global health concern. Addressing this burden demands timely and cost-effective diagnostic tools. Coronary computed tomography angiography (CCTA) is a crucial CAD assessment diagnostic modality. This non-invasive approach proves invaluable for patients with low to intermediate pre-test probabilities of ischemic heart disease, emphasizing its role in evaluating stable patients not requiring immediate revascularization.[1][2][3]
While invasive coronary angiography remains the diagnostic gold standard, CCTA steadily gains ground as a non-invasive, low-risk alternative. It circumvents the hazards associated with invasive procedures and expedites assessments for patients at intermediate risk of CAD. Given the minute dimensions and dynamic nature of epicardial coronary arteries, CCTA relies on precise spatial and temporal resolutions. Spatial resolution determines the smallest distinguishable distance between two points, while temporal resolution dictates how rapidly images of moving structures can be captured. With the advent of 64-slice multi-detector CT (64-MDCT) systems and contemporary technologies, CCTA now boasts the necessary spatial and temporal resolution to visualize even the most distal coronary artery segments.
Anatomy and Physiology
The CCTA is a radiographic procedure that allows clinicians to closely observe the heart and its various components, including the atria, ventricles, pericardium, great cardiac vessels, myocardium, and intracardiac valves. This diagnostic test involves a radiographic assessment of the epicardial coronary arteries, facilitated using a contrast agent. Although the standard cardiac CT windows may offer limited views of the adjacent pulmonary and osseous structures, the CCTA provides valuable information. A thorough understanding of coronary anatomy is imperative to interpret CT coronary angiograms accurately.
Normal Coronary Anatomy
The left main artery takes its origin from the posterior left aortic cusp. It usually measures 1 to 2 cm long and bifurcates into the left anterior descending artery (LAD) and left circumflex artery (LCx). In 0.41% of patients, the left main artery is absent, and both the LAD and LCx arise individually from the left aortic cusp. The LAD exits to the left of the pulmonary artery and travels down anteriorly in the anterior interventricular groove. Major branches from the LAD include septal perforators, which supply the anterior two-thirds of the interventricular septum, and diagonal branches, which supply the lateral wall of the left ventricle (LV). The LCx turns back into the left atrioventricular groove and gives branches called the obtuse marginal (OM), which supplies the lateral aspect of the LV. The LCx, through its course, is covered by the left auricle. In one-third of cases, the left main artery trifurcates into the LAD, LCx, and ramus intermedius, which runs between the course of LAD and LCx, supplying the anterolateral wall of the LV. The right coronary artery (RCA) originates from the anterior aspect of the right aortic cusp. The RCA runs forward into the right atrioventricular groove (AV) until the crux (a point of intersection of the right AV groove and posterior interventricular groove) divides into the posterolateral branch and the posterior descending artery (PDA). In a left-dominant system, the PDA arises from the LCx.
Indications
Studies assessing the diagnostic performance of CCTA have typically compared its ability to detect significant coronary lesions (stenoses greater than 50%) versus lesions discovered in those same patients on subsequent invasive coronary angiography. Initial studies of 64-MDCT showed a diagnostic sensitivity of 94%, specificity of 97%, positive predictive value of 87%, and negative predictive value of 99%.[4] These initial studies typically excluded patients with atrial fibrillation, atrial premature contractions, ventricular premature contractions, prior history of CAD, and an inability to tolerate beta-blockade. The reported accuracy of CCTA in chronic atrial fibrillation is a sensitivity and specificity of 95.2% and 97.6%, respectively.[5]
CCTA protocols typically involve an initial non-contrast, low-radiation dose phase. This non-contrast portion of the study can yield high-quality data about cardiac anatomical structures that may not be as adequately visualized with other noninvasive imaging modalities, eg, trans-thoracic echocardiography (TTE) or cardiac magnetic resonance imaging (MRI). Contrast images can be instrumental in diagnosing and managing adult congenital heart disease (CHD). Simple CHD includes an atrial septal defect, patent foramen ovale, ventricular septal defect, and bicuspid aortic valve. Complex CHD can also be assessed with reasonable accuracy, including Ebstein's anomaly, truncus arteriosus, hypoplastic left heart syndrome, transposition of great arteries, Tetralogy of Fallot, and tricuspid atresia. Specifically, with cases of complex CHD, many of these patients benefit from surgical repair and can survive to adulthood. CCTA provides an accurate, timely, and cost-effective means of initial diagnosis and follow-up care in these patients.
The calculation of calcium scores is a valuable function of the non-contrast portion of a CCTA, also known as an Agatston score. It is used to stratify patients into low-, intermediate-, and high-risk groups for future CAD development. While CAC scores are useful in identifying asymptomatic patients who require more intensive preventative treatment, they are rarely appropriate for symptomatic patients. Guidelines suggest that CCTA may be a more appropriate diagnostic tool for symptomatic patients with an intermediate pre-test probability of coronary disease. CAC scores are determined by assigning a weighted density score to the location of calcium with the highest attenuation, measured in Hounsfield units during the initial non-contrast phase of a CCTA, and then multiplying it by the area of calcification. CAC scores are classified into 4 risk assessment categories based on their values, which include very low risk (CAC = 0), mildly increased risk (CAC = 1–99), moderately increased risk (CAC = 100–299), and moderate to severely increased risk (CAC ≥ 300).[6] Patients with CAC scores of 0 or 1 to 10 have a very low lifetime risk of adverse cardiovascular events. However, studies have shown that patients with mild CAC scores of 1 to 10 are at 3 times the risk of developing CAD compared to patients with CAC scores of zero. These findings have led to additional investigations into the roles of non-calcified coronary artery plaque, rapid atherosclerosis, and plaque destabilization in the development of coronary heart disease. Assessing these additional plaque features via CAC scoring alone can be challenging, particularly in the case of non-calcified coronary artery plaques that can range from non-obstructive to significantly stenotic.
CCTA can also be applied to the diagnosis of coronary anomalies. As with CAD, invasive coronary angiography has been the diagnostic gold standard. However, with the temporal and spatial resolution of modern CT scanners, CCTA has emerged as a viable and strong noninvasive alternative to surveying coronary anatomy. Coronary anomalies are present in less than 1% of the population, and presentations can range from benign, incidental findings to dramatic, as in the case of sudden cardiac death. Anomalous coronary arteries are classified into 3 general groups: anomalies of origin and course, intrinsic coronary anatomy anomalies, and artery termination anomalies. In addition to assessing coronary anatomy, CCTA allows a three-dimensional assessment of the entire heart and spatial arrangements of anomalous coronaries, which can, in turn, provide prognostic information. Newer CCTA applications in perfusion and fractional flow reserve areas are on the horizon and are set to expand the diagnostic utility of cardiac CT. Additionally, transcatheter structural invasive procedures are now routinely performed, and these procedures are guided by noninvasive cardiac imaging, specifically with CCTA pre-procedurally.[7][8][9][10]
As per the Society of Cardiovascular Computed Tomography 2021 Expert Consensus Document on Coronary Computed Tomographic Angiography, the following are the appropriate utilities of CCTA in patients with CAD.[11]
CCTA in native vessels for evaluation of stable coronary artery disease:
- CCTA is appropriate as a first-line investigation in patients with no known CAD, with typical stable, atypical, or angina equivalent symptoms.
- CCTA is appropriate as a first-line investigation in patients with known CAD, with typical stable, atypical, or angina equivalent symptoms.
- CCTA is appropriate for the evaluation of CAD after inconclusive functional testing.
- CCTA may be appropriate for the assessment of asymptomatic patients who are at high risk of having CAD.
- CCTA is rarely appropriate in asymptomatic low-to-intermediate risk patients or symptomatic very low-risk patients.
CCTA for evaluation of stable coronary artery disease post-revascularization:
- CCTA is appropriate in symptomatic patients with coronary stent diameters greater than 3 mm.
- CCTA may be appropriate in symptomatic patients with coronary stent diameters of less than 3 mm, especially those with thin struts measuring less than 100 micrometers in the proximal and non-bifurcating vessels.
- CCTA is appropriate for evaluating graft patency in patients with prior coronary artery bypass grafting.
- CCTA is appropriate to assess graft and other structures before redoing cardiac surgery.
CCTA for evaluation of stable coronary artery disease using fractional flow reserve or CT perfusion:
- CCTA may be appropriate to use in the functional assessment of intermediate stenosis (30%-90%) in multivessel disease to guide decisions regarding invasive coronary angiogram and revascularization.
- Adding fractional flow reserve (FFR) or stress CT adds to the overall diagnostic value of the CCTA.
CCTA for evaluation of stable CAD in miscellaneous conditions:
- Valvular heart disease and low risk for CAD
- Nonischemic cardiomyopathy and low risk for CAD
- Coronary artery anomalies
- Screening of coronary allograft vasculopathy
- Scar assessment in patients who cannot undergo cardiac MRI
- ECG gating of patients undergoing CCTA for aortic dissection and pulmonary embolism to assess CAD in men older than 45 and women older than 55
Contraindications
There are generally no absolute contraindications to performing a CCTA. However, a history of a severe anaphylactic reaction to iodinated contrast precludes a repeat contrast administration. The following are the relative contraindications.
- Acute thyroid storm
- Pregnancy
- Renal insufficiency (defined as CrCl less than 30 mL/min/1.73 m2)
- Inability to hold breath for more than 5 seconds.
- Patients on radioactive iodine therapy
- Hemodynamic instability
- Acute decompensated heart failure
- Patient's height and weight above the recommended scanner thresholds
Equipment
The Society for Cardiovascular Computed Tomography recommends that, at a minimum, a 64-slice CT scanner be used for CCTA.[11] Dual-head power injection pumps should be utilized to take advantage of biphasic and triphasic injection protocols. Digital images should be stored in the digital imaging and communications in medicine (DICOM) format. A picture archiving and communication system (PACS) should be available to allow a review of the entire image set obtained during the scan.
Personnel
The Society for Cardiovascular Computed Tomography recommends that CCTAs be performed by technologists adequately trained to use contrast injection devices and to perform cardiac CTs and CCTAs. One member of the team should be proficient in the insertion of peripheral IV catheters. During image acquisition, a team member certified in advanced cardiac life support should also be available. Finally, a clinician trained in the administration of beta-blockers and nitroglycerin should also be present during the scan. The interpreting physician should be trained in CCTA according to the respective American College of Cardiology (ACC) and American Heart Association (AHA) clinical competence statement.
Preparation
Key facts to consider for the preparation of CCTA include the following:
- The decision to proceed with a CCTA should only be made if the results will affect a patient’s clinical management or prognosis and if there is a reasonable expectation of being able to obtain interpretable images.
- Any possible contraindications should be reviewed with subsequent evaluations of the risks versus benefits.
- Informed consent should be obtained before the start of the CCTA.
- The patient should have no solid food for 4 hours before the exam. Liquid food may be continued.
- The patient should have no caffeine for 12 hours before the test.
- Preferentially, intravenous (IV) access should be obtained in the right antecubital vein with an 18-gauge catheter. This minimizes streak artifact during image acquisition and allows rapid contrast infusion at a rate of at least 5 to 7 mL/s. Hand veins should be avoided, as this typically requires a 20-gauge or smaller catheter, which can result in slower flow rates. Central lines should not be used unless rated for power injection.
- The ideal heart rate for CCTA image acquisition is 60 beats per minute or less. An oral beta-blocker is typically administered 2 hours before the test, which can be supplemented with intravenous beta-blocker administration at the time of the test. Oral metoprolol tartrate at 50 to 100 mg is typically used as a pre-medication. Alternatives include oral atenolol, IV esmolol, calcium channel blockers, and ivabradine.
- Non-steroidal anti-inflammatory drugs should be held 24 to 48 hours before the study to reduce the risk of contrast nephropathy. Glucophage should be held for 48 hours after the procedure. Consider holding phosphodiesterase inhibitors if anticipating the use of nitrates. Nitrates vasodilate coronary arteries and improve visualization of coronary arteries and stenoses when given 5 minutes before CCTA image acquisition. Typically, 400 to 800 micrograms of sublingual nitroglycerin are used for this effect.
- Ensure appropriate lead placement.
- Make the patient practice breath-holding before the test.
Technique or Treatment
Contrast Administration
During diagnostic CCTA procedures, achieving an intra-arterial opacification of around 300 Hounsfield units is generally necessary. To accomplish this, most adults require 50 to 120 mL of iodinated contrast, injected at a rate of 5 to 7 mL/s. However, for larger patients or those undergoing coronary artery bypass graft evaluation, a higher injection rate of 6 to 7 mL/s may be used due to their larger vessels compared to native coronary arteries.
Biphasic injection protocols are employed to prevent streak artifacts caused by high concentrations of contrast on the right side of the heart. This involves injecting contrast followed by saline. If images of the right heart structures are also necessary, triphasic injection protocols can be implemented, which involve sequential injections of contrast, contrast-saline, and saline.
The scanning time and infusion rate determine the total volume of contrast required. Typically, 80 mL of contrast at a rate of 5 mL/s is used for a coronary study using a biphasic injection protocol, followed by 40 mL of saline at the same rate.
Image Acquisition
The initial phase of image acquisition in CCTA entails the acquisition of scout images, conventionally obtained as low-energy scans with a tube voltage of 120 kV and an amperage of 35 mAs. Subsequently, the contrast transit time is determined, ensuring maximal opacification of coronary vessels during the scan. This is achieved through the 'test-bolus' method, where contrast enhancement in the ascending aorta is timed at the carina level, surrogate the time required for contrast to reach the coronary arteries. Following this, parameters for image acquisition are meticulously set. The 'half-scan' reconstruction algorithm, characterized by simultaneous gantry rotation and scanner bed advancement, creating a spiral trajectory (termed 'spiral' or 'helical' CT), is commonly employed. An optimal 'pitch,' representing the ratio of bed movement to gantry rotation, is chosen to avoid overlap or gaps in reconstruction. A balance is struck as higher overlapping aids in omitting undesired image sets, enabling subsequent reconstruction. However, lower pitches result in greater radiation exposure. Fast-pitch scanners reduce acquisition time and radiation exposure but are best suited for lower heart rates. The alternative mode, 'step and shoot,' maintains a static scanner bed while the gantry orbits the patient, generating a series of axial images.
Distinguishing itself with 320 detectors, a CT scanner performs cardiac imaging in a single rotation, an advancement over 64 or 128 detector systems. Two cardinal methods of cardiac image acquisition are retrospective and prospective. Retrospective acquisition involves imaging at 10% intervals throughout the cardiac cycle, employing a tube modulator to lower radiation dose during phases where high-quality reconstruction is unnecessary. The radiation exposure is maintained at 100% during phases critical for coronary artery evaluation. Tube modulation is more efficacious at dose reduction for patients with slower heart rates. Prospective reconstruction restricts radiation exposure to a predefined phase of each cardiac cycle, often at 75% duration, offering substantial radiation reduction and thus favored. Prospective electrocardiogram (ECG)-triggered acquisition aligns X-ray tube activation with mid-diastole, further minimizing exposure. Suboptimal heart rates permit end-systole utilization. Irregular rhythms or high heart rates prompt retrospective ECG gating.
Several strategies are deployed to mitigate patient radiation exposure while preserving diagnostic accuracy. Delimiting the scan range confines radiation exposure solely to the examined structures. Typically, the CCTA scan ranges from the inferior tracheal bifurcation to the lower cardiac border. Tube potential impacts the X-ray beam's energy; customary adult CCTA protocols encompass 100 kV to 120 kV. Higher potentials enhance tissue penetration while elevating radiation exposure. Tube current influences photon quantity per unit of time; augmented current diminishes image noise but heightens radiation. Anatomy-based tube current modulation adjusts current when traversing less dense tissue, like lungs, limiting exposure. ECG-based modulation reduces current during phases of pronounced cardiac motion, particularly early and mid-systole, reducing radiation but potentially compromising diagnostic sensitivity in these motion-prone phases.
Post-Processing
The reconstruction of axial data is a critical step in assessing coronary arteries, cardiac function, and non-coronary structures. It is essential to analyze and edit the rhythm to evaluate coronary health to avoid ectopic beats. The optimal phase of the cardiac cycle is then carefully selected, and the appropriate kernel and reconstruction parameters are determined. Both maximum intensity projection (MIP) and multiplanar reformats (MPR) are utilized to assess stenosis, with coronary stenosis being re-evaluated in another cardiac phase to ensure accuracy.
For patients with irregular R-R intervals, like those with atrial fibrillation, selecting a specific phase of the R-R interval can lead to the reconstruction of different cardiac cycle phases and artifacts. To address this, the ECG is edited by selecting the phase using a specific time duration from the preceding R wave, such as 200 ms. The resulting artifact from beat-to-beat variability is known as a misalignment or banding artifact.
The late-diastole phase (60%-80% of the R-R interval) is typically chosen for coronary evaluation. However, in some cases, a systolic phase (35% of the R-R interval) is selected to minimize motion artifacts, as motion is the least at this systole stage.
Complications
CCTA testing centers should be equipped and staffed to manage the rare complication of an anaphylactic response to any of the agents administered during testing. Standard oral steroids and diphenhydramine pre-test treatments should be prescribed where specific contrast allergies are noted.
CT scans utilize x-rays, an ionizing radiation that can damage cells on a molecular level. The potential for harm from radiation exposure is cumulative over a patient’s lifetime. Thus, children and young adults are particularly at risk. Organs with high cellular turnover are also at increased risk for genetic damage from ionizing radiation exposure. Radiation exposure during CCTA should be as low as reasonably achievable to obtain diagnostic results.
Clinical Significance
CCTA is a noninvasive, cost-effective, and accurate means of assessment of CAD. It offers a timely method of anatomical evaluation of coronary arteries. CCTA provides a tremendous prognostic utility. The scope of CCTA in low-risk suspected acute coronary syndrome is also expanding. Additionally, it offers an alternative explanation of symptoms by providing information regarding non-coronary components or congenital anomalies. Continued refinements of CT technology will help reduce the risk of radiation exposure to the patients.
Enhancing Healthcare Team Outcomes
Successfully implementing CCTA within patient-centered care necessitates a multifaceted approach involving a spectrum of healthcare professionals. Physicians armed with expertise in cardiovascular imaging must demonstrate proficiency in CCTA image interpretation, encompassing the identification of cardiac anatomical structures, congenital heart disease, and coronary anomalies, while adhering to ethical standards, particularly in minimizing radiation exposure. Nurses are indispensable by ensuring patient safety and comfort during the procedure, effectively communicating instructions, and managing potential contrast-related reactions. Pharmacists contribute by optimizing contrast agent usage, dosage, and safety protocols. Effective interprofessional communication between radiologists, cardiologists, and referring clinicians is pivotal in translating diagnostic findings into actionable clinical decisions. Lastly, care coordination by healthcare teams within and across specialties ensures seamless transitions from diagnosis to subsequent interventions, guaranteeing timely and appropriate patient care, thereby improving outcomes and reinforcing the integral role of interprofessional collaboration in delivering high-quality cardiac disease management.