Continuing Education Activity
The most common anterior mediastinal masses in the adult population are thymoma, teratoma, thyroid goiter, and lymphoma. This activity describes the evaluation and management of anterior mediastinal masses and reviews the role of the healthcare team in managing patients with this condition.
Objectives:
Identify the etiology of anterior mediastinal masses.
Outline the evaluation of anterior mediastinal mass.
Review the treatment and management options available for an anterior mediastinal mass.
Explain interprofessional team strategies for improving care coordination and communication to advance anterior mediastinal mass and improve outcomes.
Introduction
The mediastinum is an anatomical space between the lungs that houses the thymus, heart, large blood vessels, lymph nodes, nerves, and portions of the esophagus and trachea. It is bound anteriorly by the sternum, posteriorly by the thoracic vertebrae, superiorly by the thoracic inlet, inferiorly by the diaphragm, and laterally by the pericardial and mediastinal pleurae. It divides into anterior, middle, and posterior compartments. Mediastinal masses encompass a broad histopathological spectrum, ranging from benign to malignant. Fifty percent of mediastinal masses occur in the anterior compartment, the most common of which are thymoma, teratoma, thyroid goiter, and lymphoma. Middle mediastinal masses typically include congenital cysts, while posterior mediastinal masses are often neurogenic tumors, such as schwannomas. The focus of our discussion will center on anterior mediastinal masses.
Etiology
The anterior mediastinum contains thymus, fat, and lymph nodes, which corresponds with the most common etiologies of associated primary tumors. Although two-thirds of mediastinal masses are benign, about 59% of masses in the anterior compartment are malignant.[1] Most anterior mediastinal masses are epithelial tumors. Thymoma is the most commonly occurring anterior mediastinal mass in the adult population, although it accounts for less than one percent of adult malignancies.[2] Benign masses of thymic origin are rare but include thymolipomas, which are characterized by adipose composition, as well as thymic cysts. Thymic cysts may be congenital, which represent remnants of the thymopharyngeal duct or acquired, as a manifestation of an inflammatory neoplasm like Hodgkin lymphoma.[1] To juxtapose, thymic masses may rarely present as aggressive and invasive neoplasms in the forms of thymic carcinoma and thymic carcinoid. Importantly, thymic masses require differentiation from thymic hyperplasia, which further subdivides into true and lymphoid (follicular) types. The definition of true thymic hyperplasia is an enlarged thymus with the retained original shape and typically occurs with stressful conditions, such as chemotherapy, burns, and corticosteroid administration. Lymphoid thymic hyperplasia results from an increased number of lymphoid follicles within the gland and is associated with autoimmune conditions, such as myasthenia gravis.[2]
Germ cell tumors (GCTs) contain primitive germ cells that fail to migrate entirely during embryonic development. Although they mainly arise in the gonads, germ cell tumors represent 15% of anterior mediastinal masses.[1] The most common germ cell tumors are benign teratomas, which, by definition, contain tissues from at least two of the three germ layers. Benign teratomas most commonly contain ectodermic tissue, such as hair and teeth, but may also contain tissues derived from mesoderm and endoderm, such as bone and intestinal epithelium, respectfully. If a teratoma contains fetal or neuroendocrine tissue, it is classified as immature and malignant and carries a poor prognosis.[2] Seminomas and non-seminomatous germ cell tumors (NSGCT) are rare malignant GCTs that usually present as bulky, large, lobulated masses. Seminomas represent 25 to 50% of malignant GCT.[2] Non-seminomatous germ cell tumors include yolk sac tumors, endodermal sinus tumors, embryonal carcinomas, choriocarcinomas, and mixed germ cell tumors. These tumors are classically associated with elevated levels of alpha-fetoprotein (AFP) and beta-human chorionic gonadotropin (beta-hCG).
Primary mediastinal lymphomas comprise 10% of all mediastinal lymphomas.[2] Hodgkin lymphoma (HD) accounts for 50% to 70% of primary lymphomas and further subdivides into the following subtypes: nodular sclerosing (most common), lymphocyte-rich, lymphocyte-depleted, and mixed-cellularity. Non-Hodgkin lymphoma (NHL) comprises 15 to 25% of primary lymphomas, the most common of which are diffuse large B-cell non-Hodgkin lymphoma, also referred to as mediastinal large cell non-Hodgkin lymphoma (MLC-NHL), and lymphoblastic non-Hodgkin lymphoma (LB-NHL).[2]
Another cause of anterior mediastinal mass is mediastinal goiter. Mediastinal goiters typically arise when the lower part of the cervical thyroid lobes extend into the thoracic inlet. Less commonly, mediastinal goiters represent remnants of embryonic tissues.[3] Other even less common causes of anterior mediastinal masses include parathyroid adenomas, hemangiomas, and sarcomas. Fibrosing mediastinitis, which is characterized by progressive infiltration of fibrous tissue, is additionally another unusual cause of anterior mediastinal masses. Etiologies for fibrosing mediastinitis include infections, such as tuberculosis and histoplasmosis, as well as reactions to autoimmune syndromes, drugs, and radiation.[4]
Epidemiology
The most frequent etiologies of anterior mediastinal masses are thymic malignancies and lymphoma, with approximate incidences of 35% and 25%, respectively. Less common causes of anterior mediastinal masses are thyroid and endocrine tumors (incidence of 15%), benign teratomas (incidence of 10%), malignant germ cell tumors (incidence of 10%), and benign thymic lesions (incidence of 5%).[5] When evaluating the epidemiology of anterior mediastinal masses, patient age and gender are the most important determinants. For both men and women greater than 40 years of age, thyroid goiter and thymic malignancies represent two-thirds of anterior mediastinal masses. Between thirty and fifty percent of patients with thymomas will have an associated paraneoplastic syndrome, the most common of which is myasthenia gravis (MG), followed by hypogammaglobulinemia and pure red cell aplasia.[5]
In females between 10 to 39 years of age, the most common proportion of mediastinal masses are lymphomas, most typically Hodgkin lymphoma and mediastinal large cell non-Hodgkin lymphoma.[5] Hodgkin lymphoma notably has a classic bimodal distribution of incidence, which peaks in young adulthood and at ages greater than 50 years. The next most common etiologies of anterior mediastinal masses in this population are thymic malignancies, which often present in women aged greater than 20 years of age and benign teratomas, which is more prevalent in females of less than 25 years.[5] In contrast, men in the age range of 10 to 39 years, as well as children less than 10 years of age, have no dominant tumor type of the anterior mediastinum.[2] The diagnosis of anterior mediastinal mass in these populations largely relies on the rapidity of symptom manifestation, as well as characteristic radiologic features.
History and Physical
The diagnosis of anterior mediastinal mass may be an incidental finding in the asymptomatic patient. However, 60% of patients are symptomatic at presentation.[6] Moreover, symptoms usually correlate with a malignant process. Symptoms categorize into localizing or pressure-induced, which are secondary to tumor invasion and systemic, which are the result of the release of hormones, antibodies, and cytokines. The most common localizing symptom is cough, which is present 60% of the time.[1] Other common obstructive symptoms include chest pain, dyspnea, voice hoarseness, hemoptysis, and dysphagia. Bulky mediastinal tumors, such as germ cell tumors, may cause compression of the superior vena cava (SVC), which drains the head, upper extremities, and upper thorax. Superior vena cava syndrome is associated with elevated intracranial venous pressures, which may result in periorbital and facial edema, proptosis, facial plethora, and upper extremity swelling, in addition to the more common localizing symptoms.[7] When a tumor is large enough to provoke injury to the oculosympathetic pathway, Horner syndrome may occur, resulting in the classic triad of unilateral miosis, eyelid ptosis, and facial anhidrosis.[8]
Manifestations of paraneoplastic syndromes can occur with anterior mediastinal masses, especially thymomas. The constellation of ptosis, dysphagia, weakness, and fatigue point to the most commonly associated paraneoplastic syndrome, myasthenia gravis. Thirty to 50% of patients with thymoma have MG, while 10% to 15% of patients with MG have thymoma.[1] Non-specific symptoms of anemia, such as fatigue, will be present with the autoimmune-mediated paraneoplastic syndrome known as pure red cell aplasia, which is associated with thymoma and lymphoma. A predisposition to recurrent infections may hint to hypogammaglobulinemia, which also has links with thymoma. The constitutional B-symptoms of fevers, night sweats, and weight loss are classically seen with lymphoma, but may also be present with other malignancies. Pain after alcohol ingestion, although rare, is specific for Hodgkin lymphoma. Symptoms of hypercalcemia, such as abdominal pain and altered mental status, may be present with lymphoma and parathyroid adenomas. Thyrotoxicosis may point to a diagnosis of thyroid goiter. It is also crucial to assess the rapidity of symptom manifestations. Rapid onset of symptoms would suggest an aggressive tumor, such as lymphoma or germ cell tumor, while a more indolent course may represent a slower-growing tumor, such as thymoma or teratoma.
It is essential to perform a thorough physical exam. When looking at the patient, evaluate for ptosis, proptosis, or signs of facial edema. Voice hoarseness may prompt further evaluation of the vocal cords and upper airway with a laryngoscopy. Palpation of the cervical, supraclavicular, and axillary lymph nodes may reveal palpable bulky adenopathy. However, palpation of the lymph node regions is imperative, as sites distal to the mediastinum may also be involved. Heart auscultation may reveal a subtle pericardial rub, which would suggest a pericardial effusion. Lung exam may reveal crackles, which would suggest a possible pleural effusion. Bronchial breathing over the thoracic vertebrae is a positive d’Espine sign, which correlates with mediastinal lymphadenopathy. Gynecomastia may occur from the release of beta-hCG, as is common with non-seminomatous germ cell tumors.[1] An abdominal exam should evaluate for hepatosplenomegaly. Attention should be devoted to weakness and cranial nerve deficits on the neurologic exam.
Evaluation
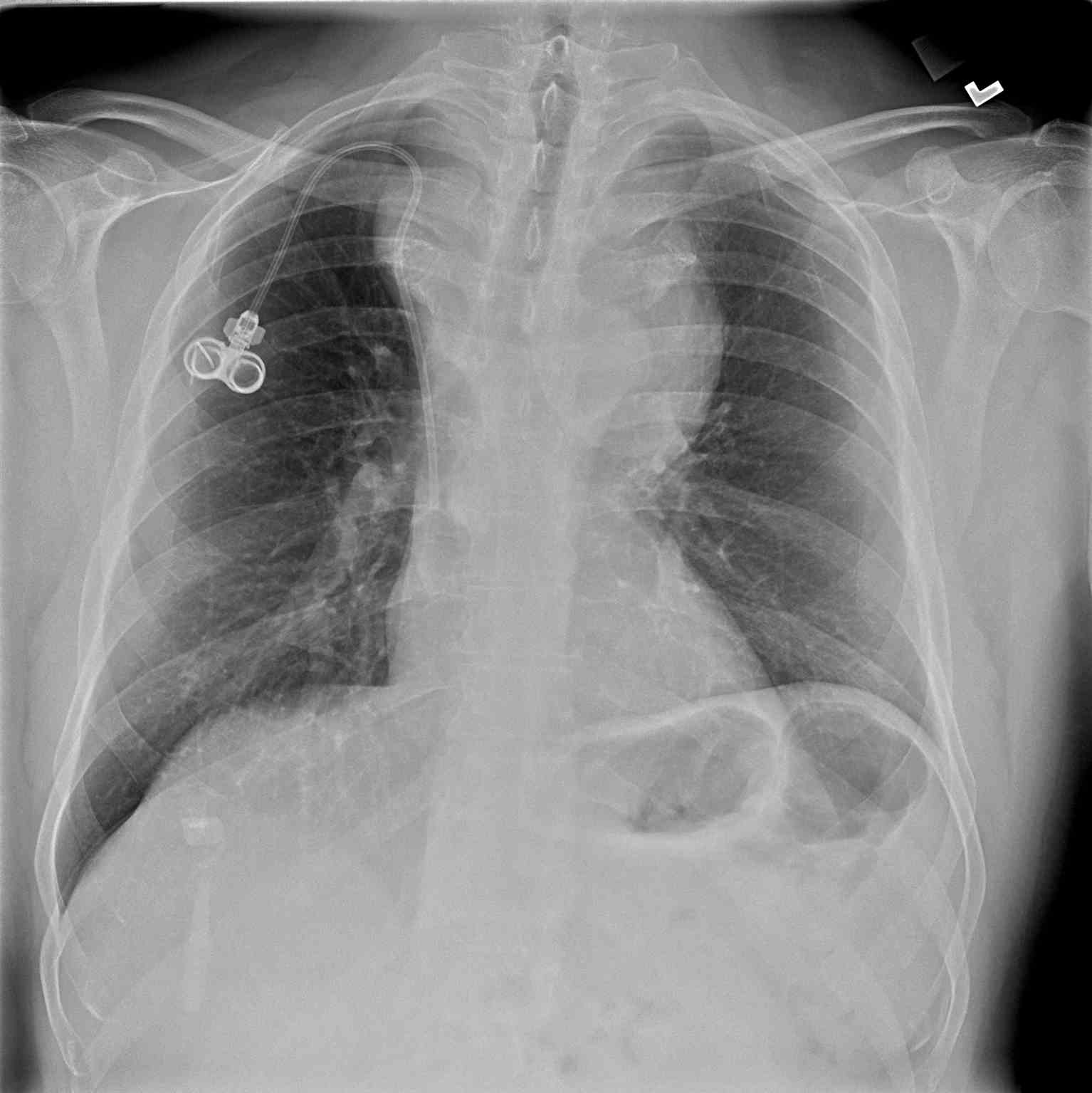
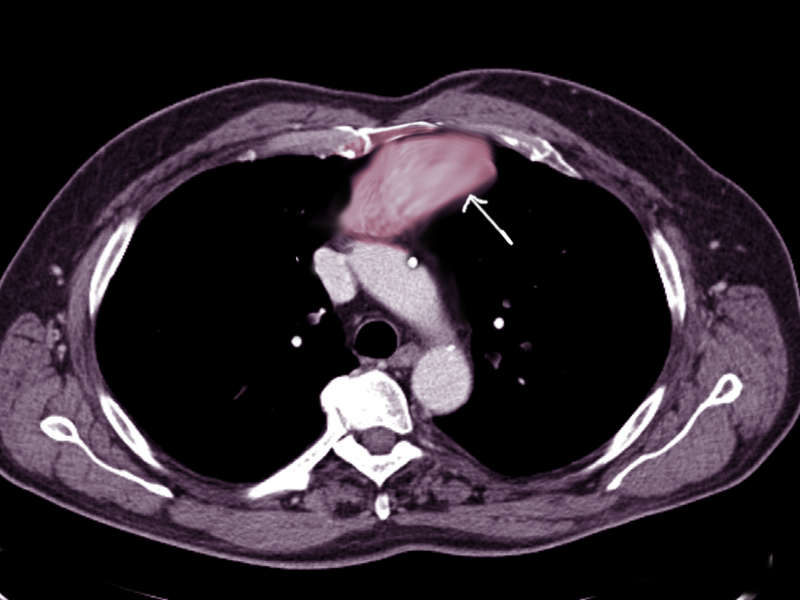
Chest imaging plays a crucial role in the evaluation of anterior mediastinal masses. The initial detection of anterior mediastinal masses may occur on chest radiographs (CXR); however, basic anatomical structures may not be visible. Chest computed tomography (CT) with intravenous contrast is the most important radiologic tool for evaluation, as it depicts different tissue attenuations.[2] For example, a thymoma will appear as a slightly heterogeneous mass with smooth or lobular margins, while a teratoma will typically be cystic and contain varying amounts of fat, soft tissue, and calcification. CT not only helps characterize lesions but also aids with the staging of disease processes. Thus, mediastinal lymph node enlargement merits special attention. The presence of pleural or pericardial effusions on chest imaging also requires assessment, as this can clue the physician into the diagnosis of lymphoma or non-seminomatous germ cell tumors.[2] Positron emission tomography with computed tomography (PET-CT) is a more useful modality for staging lymphomas, as it is more accurate at detecting both intra-nodal and extranodal disease than CT.[1] Magnetic resonance imaging (MRI) is not always a necessary part of the work-up but is useful in some instances, such as distinguishing thymic hyperplasia from thymic malignancy.[2] For example, on MRI, a thymic neoplasm may appear as a focal, nodular mass with a necrotic and calcified center, while thymic hyperplasia will be smooth and symmetric in morphology. Additionally, MRI helps confirm the presence of fat, which is also more suggestive of thymic hyperplasia.[2] MRI without contrast for the evaluation of anterior mediastinal masses is an option; therefore, for patients with a contraindication to intravenous contrast, it is the diagnostic imaging modality of choice.
Additional imaging tests may be necessary for specific etiologies of anterior mediastinal masses. When evaluating a mediastinal goiter for active thyroid tissue, scintigraphy with radioactive iodine will be useful. If a mediastinal parathyroid adenoma is suspected, nuclear scans with isotopes, such as technetium-99, should be ordered. All male patients with a mediastinal germ cell tumor should undergo testicular ultrasound as part of a thorough work-up for primary gonadal malignancy. Pulmonary function tests are necessary for all patients. Spirometry will typically be normal; however, flattening of the inspiratory flow loop on the flow-volume curve may be identified in cases of variable extrathoracic obstruction caused by the tumor.
The clinician should obtain a complete blood count and complete metabolic panel in all patients. Additionally, AFP and beta-hCG are necessary if there is a high suspicion for non-seminomatous germ cell tumors, as levels will typically present as elevated. Lactate dehydrogenase (LDH) is less specific but is usually also increased with non-seminomatous germ cell tumors, as well as lymphoma. Depending on the clinical picture, the clinician may consider additional labs, such as a thyroid panel, if concerned for thyrotoxicosis. Thyroid carcinoid is associated with Cushing syndrome; therefore, the clinician should pursue a hypercortisolism workup in those cases. When suspecting a diagnosis of myasthenia gravis, antibodies against acetylcholine receptors, muscle-specific kinase (MuSK), and lipoprotein receptor-related protein 4 (LRP4) are in order. It should be noted, however, that 10 to 15% of patients with clinical MG will be seronegative for commercially tested antibodies due to insufficiently sensitive tests, or the presence of antibodies against other postsynaptic membrane antigens.[9] Gammaglobulins (IgG, IgA, and IgM) are also diagnostic, as hypogammaglobulinemia is a paraneoplastic syndrome associated with thymoma.
A diagnostic biopsy is obtainable via transthoracic or transbronchial needle aspiration, mediastinoscopy, anterior mediastinotomy, or video-assisted thoracoscopic surgery (VATS). However, a tissue biopsy is not always necessary for the diagnosis of an anterior mediastinal mass when the clinical picture and the radiographic characteristics fit a classic prototype, as is typically the case with thymoma, teratoma, and thyroid goiter. For example, reliable diagnosis of thymoma can be made in an adult older than 50 years of age with MG and the typical appearance of thymoma on CT. Needle biopsy of thymoma can prove to have adverse effects, such as tumor seeding, which case reports have described.[10]
In the case of lymphoma, tissue is needed to make the diagnosis and characterize the tumor, as some subtypes are manageable purely non-surgically. Although fine-needle biopsy may be initially employed, the yield is not always reliable for the dense, fibrotic lymphomas like Hodgkin lymphoma and mediastinal large cell non-Hodgkin lymphoma. To sufficiently define the characteristics of an anterior mediastinal lymphoma, multiple core biopsies (3 to 5) are often necessary for immunohistochemistry, as well as the fine-needle aspiration for flow cytometry.[5] Still, when the biopsy results and clinical picture conflict, larger tissue specimens will be necessary, obtained via CT-guided transthoracic needle biopsy, VATS, mediastinotomy, or open surgical biopsy. Whenever obtaining diagnostic tissue, pathology should confirm that there is adequate tissue for examination. Rapid on-site cytological examination (ROSE) involves rapid tissue evaluation by pathology while the patient is still under procedural sedation so that additional tissue can be obtained if necessary, rather than submitting the patient for an added procedure.[11]
Treatment / Management
Surgical resection is the treatment of choice for a majority of anterior mediastinal masses, including thymomas, thymic carcinomas, thymic carcinoids, mediastinal goiters, and parathyroid adenomas. In cases of thymomas, adjunctive chemotherapy and radiation are options for locally invasive or metastatic disease, and inoperable tumors.[1] In cases that are surgically-resectable, induction chemotherapy before surgery, followed by consolidation chemotherapy and radiation after surgery, correlates with an improved 5-year survival rate of 95%. The typical induction regimen consists of three cycles of cyclophosphamide, doxorubicin, cisplatin, and prednisone, while the consolidation regimen incorporates three cycles of the same drugs at 80% of the doses.[12] For advanced non-resectable thymic carcinomas, chemotherapy regimens consisting of cisplatin, vincristine, doxorubicin, and etoposide have resulted in a median survival period of 49 months.[13] Although often employed for thymic carcinoid tumors, chemotherapy has been shown to have minimal effects in these cases. Thymic cysts, although usually benign, should be surgically excised because they may be microscopically identical to thymic neoplasms.[1] Thymic hyperplasia may be observed or resected when symptomatic, as in the cases of severe MG.
Teratomas are usually benign; therefore, resection is indicated when the lesions become symptomatic. Seminomas are generally responsive to radiation therapy, but chemotherapy and surgical resection may also be indicated depending on the disease extent. Non-seminomatous germ cell tumors receive a standard chemotherapy regimen of bleomycin, etoposide, and cisplatin. Patients with residual tumor following chemotherapy may undergo surgical resection.[1]
The direction of treatment for Hodgkin lymphoma depends on the stage of the disease. For disease in stages I and II, the standard treatment involves field radiation and combination chemotherapy with epirubicin, bleomycin, vinblastine, and prednisone. Stage III and IV Hodgkin lymphoma primarily receive treatment with only chemotherapy. The standard of care regimen known as ABVD consists of doxorubicin (adriamycin), bleomycin, vinblastine, and dacarbazine.[1] Patients with Hodgkin lymphoma who relapse may benefit from a bone marrow transplant (BMT), with autologous being superior to allogeneic due to decreased mortality (27% versus 48%).[14]
The treatment of mediastinal non-Hodgkin lymphoma is typically non-surgical. Due to its propensity to involve the bone marrow and its high relapse rate, the treatment of lymphoblastic non-Hodgkin lymphoma consists of intensive chemotherapy with a maintenance phase chemotherapy. Additionally, patients with lymphoblastic non-Hodgkin lymphoma typically receive intrathecal chemotherapy and brain irradiation to prevent central nervous system relapse.[1] Patients with mediastinal large cell non-Hodgkin lymphoma usually have treatment with conventional chemotherapy and radiation. For both disease entities, a bone marrow transplant is indicated in cases of relapse with traditional therapy.
Differential Diagnosis
- Lymphadenopathy
- Bronchogenic cyst
- Pericardial cyst
- Enteric cyst
- Thoracic aortic aneurysm
- Esophageal tumor
- Lymphangioma
- Neurogenic tumor
- Meningocele
- Thoracic spine lesion
Staging
The two staging systems worth mentioning are the Masaoka-Koga staging system for thymoma and the Ann-Arbor staging system with Cotswold modifications for Hodgkin lymphoma.
The Masaoka-Koga staging system for thymomas has as its basis the local extension of the tumor. Stage I represents an encapsulated tumor. Stage IIA and IIB represent invasion through the capsule and into the surrounding fatty tissue, respectively. Invasion into neighboring organs, such as the pericardium, is denoted by stage III. Stage IVA and IVB encompass pleural or pericardial metastasis and lymphatic-hematologic metastasis, respectively.
The Ann-Arbor staging system with Cotswold modifications has classically been used to stage Hodgkin lymphoma. Stage I disease involves one lymph node region. Stage II disease involves two or more lymph node regions on the same side of the diaphragm. Lymph node regions on both sides of the diaphragm denote stage III disease. Stage IV Hodgkin lymphoma encompasses involvement at extranodal sites. In addition to staging, other disease characteristics, or modifications, must be specified. The Ann-Arbor Cotswold modifications include the absence of symptoms (A), presence of B-symptoms (B), bulky disease denoted as greater than 10 cm in diameter (X), and involvement of a single, contiguous, or extranodal site (E).
Prognosis
Complete surgical resection is the factor that most correlates with thymoma prognosis.[2] The Masaoka-Koga staging system has been used to estimate 5-year survival. Encapsulated tumors (stage I) carry the best prognosis, with a 96 to 100% 5-year survival rate, while metastatic disease (stages IVA and IVB) naturally carry the worst prognosis, with 5-year survival rates of 11% to 50%.[1] Other predictors of poor prognosis include tumor size greater than 10 centimeters, age less than 30 years, tracheal or vascular compression, epithelial or mixed histology, and the presence of a paraneoplastic syndrome.[1] In contrast to thymoma, the diagnosis of thymic carcinoma and carcinoid carry a much poorer prognosis. Thus, the Masaoka-Koga staging system is not a useful prognostic tool. Cytologic features that portend a poor prognosis include high-grade atypia, necrosis, and greater than ten mitoses per high-power field.[1]
Of germ cell tumors, teratomas carry the best prognosis, as most of them are benign. Seminomas and non-seminomatous germ cell tumors have a poorer prognosis and have 5-year survival rates of 86% and 48%, respectively. Persistently elevated tumor-markers (AFP and beta-hCG) after chemotherapy is associated with worse outcomes.[1]
Stage I and II Hodgkin disease patients have a favorable prognosis, with greater than 90% cure rates with standard treatment. Stage IIIA and IIIB have cure rates of 60% to 70%. Stage IV disease carries the worst prognosis, with a cure rate of 50% to 60%. [C] Poor prognostic factors for Hodgkin lymphoma include the following at diagnosis: male gender, age greater than 45 years, serum albumin less than 4 g/dL, hemoglobin less than 10.5 g/dL, and white blood cell count greater than or equal to 15000 cells/microliter, and lymphocyte count of less than 600 cells/microliter.[15]
Complications
Among the most feared complications of anterior mediastinal masses is the invasion of tumor and metastasis, which naturally foreshadows a poorer prognosis. Additionally, invasive and bulky tumors may present with a variable extrathoracic obstruction, which may result in airway compromise and compression symptoms, such as superior vena cava syndrome and Horner syndrome. If there is true airway compromise, the patient may have to undergo a tracheostomy. Many of the complications associated with anterior mediastinal masses are related to the treatment options. Certainly, any surgical intervention carries risks and related morbidity. Exposure to chemotherapy and radiation have side effects, such as bone marrow suppression and an increased chance of malignancy, respectively.
Deterrence and Patient Education
Patients must receive education on their specific disease process, the treatment options, and the prognosis. Patients should be actively involved throughout the entirety of the treatment course and should be encouraged to have consistent follow up due to the possibility of disease relapse.
Enhancing Healthcare Team Outcomes
Clinicians may rarely encounter anterior mediastinal masses in their daily practices. Many times anterior mediastinal masses will be incidental findings on radiographic studies. For this reason, clinicians must take the time to review radiologic images, rather than depend on radiologists’ reports. After diagnosing a patient with an anterior mediastinal mass, he or she will encounter various healthcare professionals along the treatment course. Patient care should involve an interprofessional team.
Depending on the diagnosis, patients may consult with endocrinologists, oncologists, pulmonologists, otolaryngologists, and cardiothoracic surgeons. Oncology-specialty pharmacists will play a role in chemotherapy dosing and can verify agent selection, as well as guarding against any potential drug-drug interactions. They will report any concerns to the managing clinician and the rest of the team. Specialty-trained oncology nurses are also crucial members of the interprofessional team, as they will have more ongoing and frequent contact with the patient, administer medications, and be in a position to monitor for adverse effects as well as treatment effectiveness, which they must communicate appropriately.
Speech and language pathologists may be involved in the care of patients with voice changes or difficulty swallowing. Ancillary staff should have training on typical signs and symptoms of the disease, as well as potential complications. Mediastinal masses, while rare, still require the collaboration of an interprofessional team approach to direct patient outcomes successfully. [Level V]