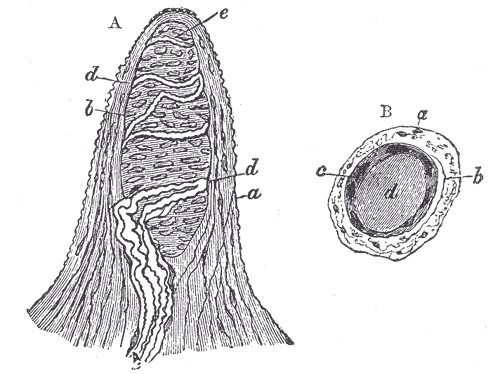
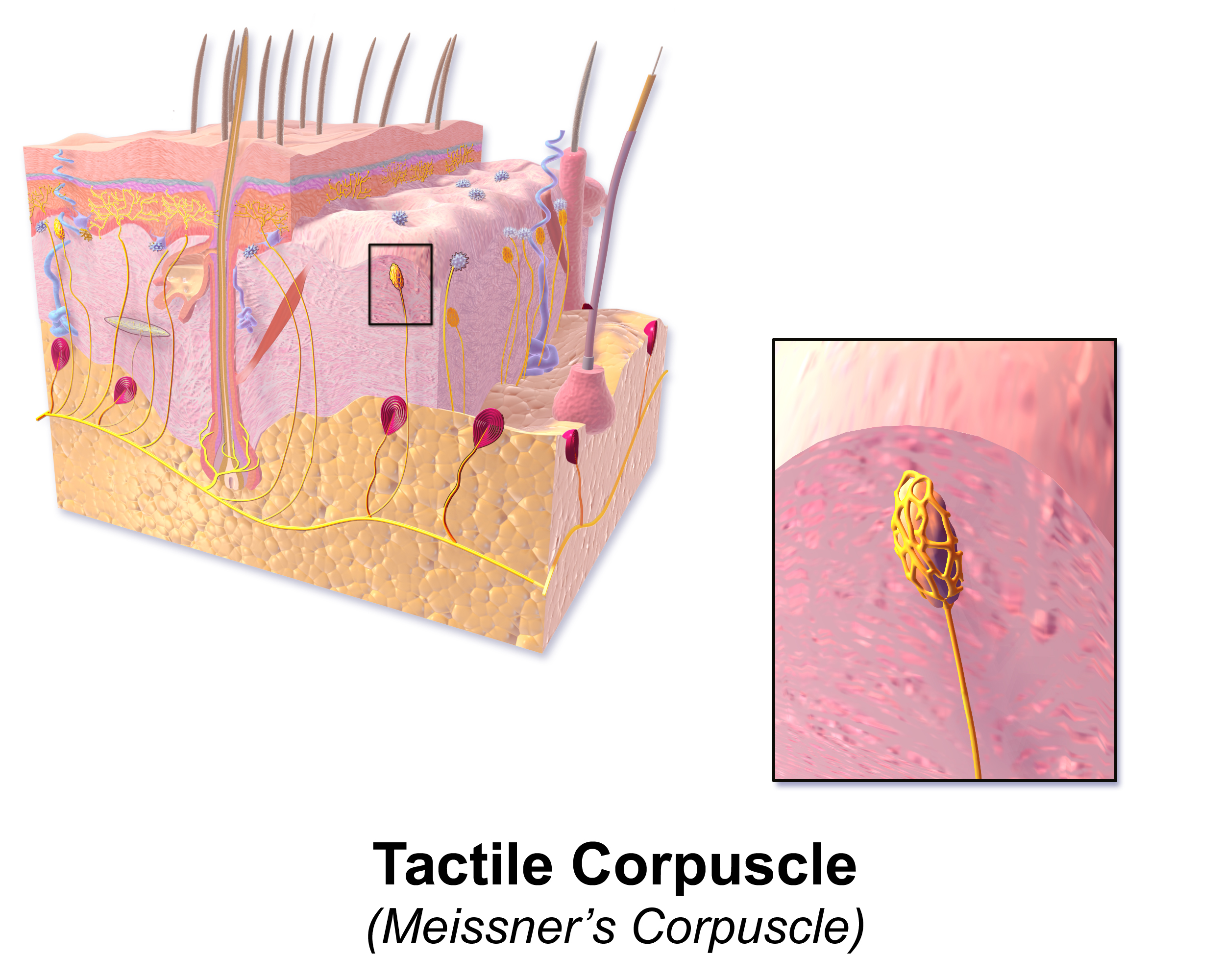
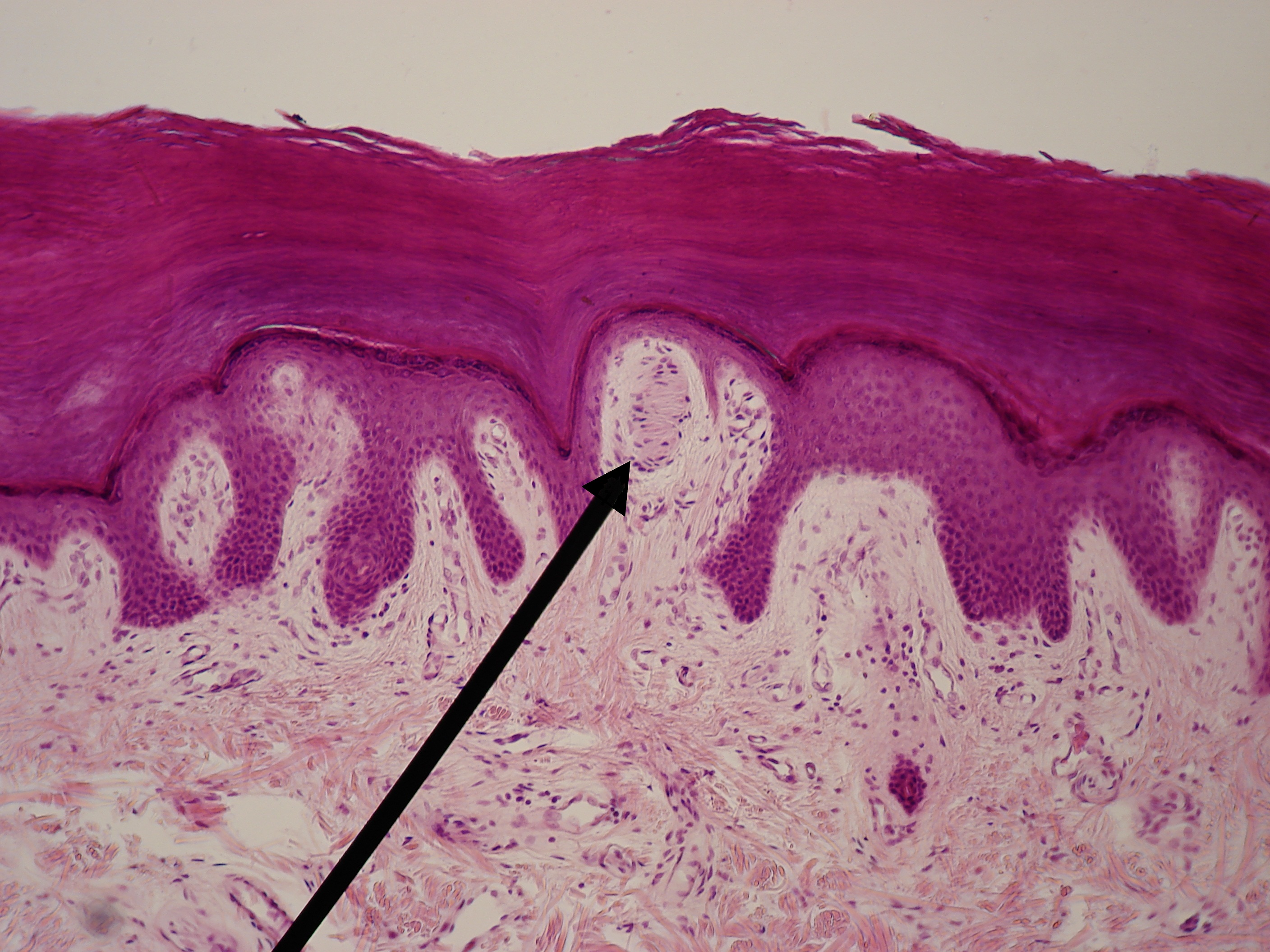
[1]
Vega JA, López-Muñiz A, Calavia MG, García-Suárez O, Cobo J, Otero J, Arias-Carrión O, Pérez-Piñera P, Menéndez-González M. Clinical implication of Meissner`s corpuscles. CNS & neurological disorders drug targets. 2012 Nov 1:11(7):856-68
[PubMed PMID: 23131158]
[2]
CAUNA N, ROSS LL. The fine structure of Meissner's touch corpuscles of human fingers. The Journal of biophysical and biochemical cytology. 1960 Oct:8(2):467-82
[PubMed PMID: 13691669]
[3]
Idé C. The fine structure of the digital corpuscle of the mouse toe pad, with special reference to nerve fibers. The American journal of anatomy. 1976 Nov:147(3):329-55
[PubMed PMID: 983972]
[4]
Vega JA, García-Suárez O, Montaño JA, Pardo B, Cobo JM. The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microscopy research and technique. 2009 Apr:72(4):299-309. doi: 10.1002/jemt.20651. Epub
[PubMed PMID: 19012318]
[5]
CAUNA N. Nerve supply and nerve endings in Meissner's corpuscles. The American journal of anatomy. 1956 Sep:99(2):315-50
[PubMed PMID: 13372495]
[6]
Zimmerman A, Bai L, Ginty DD. The gentle touch receptors of mammalian skin. Science (New York, N.Y.). 2014 Nov 21:346(6212):950-4. doi: 10.1126/science.1254229. Epub
[PubMed PMID: 25414303]
[7]
González-Martínez T, Fariñas I, Del Valle ME, Feito J, Germanà G, Cobo J, Vega JA. BDNF, but not NT-4, is necessary for normal development of Meissner corpuscles. Neuroscience letters. 2005 Mar 22:377(1):12-5
[PubMed PMID: 15722178]
Level 3 (low-level) evidence
[8]
González-Martínez T, Germanà GP, Monjil DF, Silos-Santiago I, de Carlos F, Germanà G, Cobo J, Vega JA. Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain research. 2004 Mar 26:1002(1-2):120-8
[PubMed PMID: 14988041]
[9]
Ichikawa H, Matsuo S, Silos-Santiago I, Sugimoto T. Developmental dependency of Meissner corpuscles on trkB but not trkA or trkC. Neuroreport. 2000 Feb 7:11(2):259-62
[PubMed PMID: 10674466]
[10]
Paré M, Elde R, Mazurkiewicz JE, Smith AM, Rice FL. The Meissner corpuscle revised: a multiafferented mechanoreceptor with nociceptor immunochemical properties. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2001 Sep 15:21(18):7236-46
[PubMed PMID: 11549734]
[11]
Hoffmann JN, Montag AG, Dominy NJ. Meissner corpuscles and somatosensory acuity: the prehensile appendages of primates and elephants. The anatomical record. Part A, Discoveries in molecular, cellular, and evolutionary biology. 2004 Nov:281(1):1138-47
[PubMed PMID: 15470674]
[12]
Herrmann DN, Boger JN, Jansen C, Alessi-Fox C. In vivo confocal microscopy of Meissner corpuscles as a measure of sensory neuropathy. Neurology. 2007 Dec 4:69(23):2121-7
[PubMed PMID: 17898322]
[13]
Castano P, Rumio C, Morini M, Miani A Jr, Castano SM. Three-dimensional reconstruction of the Meissner corpuscle of man, after silver impregnation and immunofluorescence with PGP 9.5 antibodies using confocal scanning laser microscopy. Journal of anatomy. 1995 Apr:186 ( Pt 2)(Pt 2):261-70
[PubMed PMID: 7649825]
[14]
Pham TQ, Hoshi T, Tanaka Y, Sano A, Kawaue T, Miyata T. Two-Photon Imaging of DiO-Labelled Meissner Corpuscle in Living Mouse's Fingertip. IEEE transactions on haptics. 2016 Oct-Dec:9(4):483-491
[PubMed PMID: 27254872]
[15]
Vega JA, Llamosas MM, Huerta JJ, García-Fernández JM. Study of human cutaneous sensory corpuscles using double immunolabelling and confocal laser scanning microscopy. The Anatomical record. 1996 Dec:246(4):557-60
[PubMed PMID: 8955795]
[16]
Takahashi-Iwanaga H, Shimoda H. The three-dimensional microanatomy of Meissner corpuscles in monkey palmar skin. Journal of neurocytology. 2003 May:32(4):363-71
[PubMed PMID: 14724379]
[17]
Fellegara G, Young RH, Kuhn E, Rosai J. Ovarian mature cystic teratoma with florid vascular proliferation and Wagner-Meissner--like corpuscles. International journal of surgical pathology. 2008 Jul:16(3):320-3. doi: 10.1177/1066896907307306. Epub
[PubMed PMID: 18573789]
[18]
Kaiserling E, Geerts ML. Tumour of Wagner-Meissner touch corpuscles. Wagner-Meissner neurilemmoma. Virchows Archiv. A, Pathological anatomy and histopathology. 1986:409(2):241-50
[PubMed PMID: 3087055]
[19]
Wu AJ, Jarzembowski J, Morag Y, Lucas DR. Wagner-Meissner neurilemmoma of the right cheek. Annals of diagnostic pathology. 2008 Jun:12(3):204-7. doi: 10.1016/j.anndiagpath.2006.09.001. Epub 2007 Aug 20
[PubMed PMID: 18486897]
[20]
Ferrara N, Di Marino M, Rossiello L, Baldi A. Wagner-Meissner neurilemmoma of the vulva. International journal of dermatology. 2003 Jul:42(7):550-1
[PubMed PMID: 12839606]
[21]
Huber AR, Agostini-Vulaj D, Drage MG, Lemmon JW. Tactile Corpuscle-Like Bodies (Wagner-Meissner Corpuscles) of the Colorectum: A Series of 5 Cases. International journal of surgical pathology. 2017 Dec:25(8):684-687. doi: 10.1177/1066896917723982. Epub 2017 Aug 7
[PubMed PMID: 28784007]
Level 3 (low-level) evidence
[22]
Celeiro-Muñoz C, Huebner TA, Robertson SA, Pittman ME, Singhi AD, Arnold CA, Bhaijee F, Voltaggio L, Montgomery EA. Tactile Corpuscle-like Bodies in Gastrointestinal-type Mucosa: A Case Series. The American journal of surgical pathology. 2015 Dec:39(12):1668-72. doi: 10.1097/PAS.0000000000000480. Epub
[PubMed PMID: 26291509]
Level 2 (mid-level) evidence
[23]
Wills EJ, Croker J, Brammah S. Tactile corpuscle-like bodies in colonic mucosa. Ultrastructural pathology. 2003 Mar-Apr:27(2):79-86
[PubMed PMID: 12746198]
[24]
Dillon YK, Haynes J, Henneberg M. The relationship of the number of Meissner's corpuscles to dermatoglyphic characters and finger size. Journal of anatomy. 2001 Nov:199(Pt 5):577-84
[PubMed PMID: 11760888]
[25]
Iwasaki T, Goto N, Goto J, Ezure H, Moriyama H. The aging of human Meissner's corpuscles as evidenced by parallel sectioning. Okajimas folia anatomica Japonica. 2003 Mar:79(6):185-9
[PubMed PMID: 12776944]
[26]
Nava PB, Mathewson RC. Effect of age on the structure of Meissner corpuscles in murine digital pads. Microscopy research and technique. 1996 Jul 1:34(4):376-89
[PubMed PMID: 8807620]
[27]
Mathewson RC, Nava PB. Effects of age on Meissner corpuscles: a study of silver-impregnated neurites in mouse digital pads. The Journal of comparative neurology. 1985 Jan 8:231(2):250-9
[PubMed PMID: 3968237]
Level 2 (mid-level) evidence
[28]
Nolano M, Provitera V, Estraneo A, Selim MM, Caporaso G, Stancanelli A, Saltalamacchia AM, Lanzillo B, Santoro L. Sensory deficit in Parkinson's disease: evidence of a cutaneous denervation. Brain : a journal of neurology. 2008 Jul:131(Pt 7):1903-11. doi: 10.1093/brain/awn102. Epub 2008 May 31
[PubMed PMID: 18515869]
[29]
Almodovar JL, Ferguson M, McDermott MP, Lewis RA, Shy ME, Herrmann DN. In vivo confocal microscopy of Meissner corpuscles as a novel sensory measure in CMT1A. Journal of the peripheral nervous system : JPNS. 2011 Sep:16(3):169-74. doi: 10.1111/j.1529-8027.2011.00342.x. Epub
[PubMed PMID: 22003930]
[30]
Almodovar JL, Schifitto G, McDermott MP, Ferguson M, Herrmann DN. HIV neuropathy: an in vivo confocal microscopic study. Journal of neurovirology. 2012 Dec:18(6):503-10. doi: 10.1007/s13365-012-0130-1. Epub 2012 Oct 16
[PubMed PMID: 23070817]
[31]
Shun CT, Chang YC, Wu HP, Hsieh SC, Lin WM, Lin YH, Tai TY, Hsieh ST. Skin denervation in type 2 diabetes: correlations with diabetic duration and functional impairments. Brain : a journal of neurology. 2004 Jul:127(Pt 7):1593-605
[PubMed PMID: 15128619]
[32]
Albuerne M, López S, Naves FJ, Martínez-Almagro A, Represa J, Vega JA. S100alpha and S100beta proteins in human cutaneous sensory corpuscles: effects of nerve and spinal cord injury. The Anatomical record. 1998 Jul:251(3):351-9
[PubMed PMID: 9669763]
[33]
Márquez J, Pérez-Pérez M, Naves FJ, Vega JA. Effect of spinal cord and peripheral nerve injury on human cutaneous sensory corpuscles. An immunohistochemical study. Journal of the peripheral nervous system : JPNS. 1997:2(1):49-59
[PubMed PMID: 10975736]