Introduction
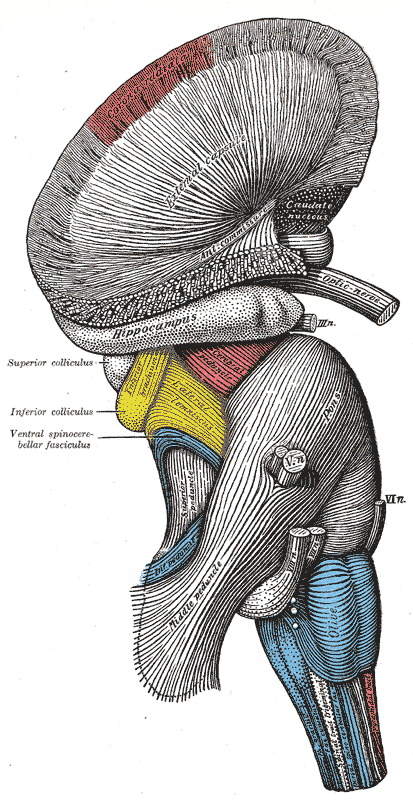
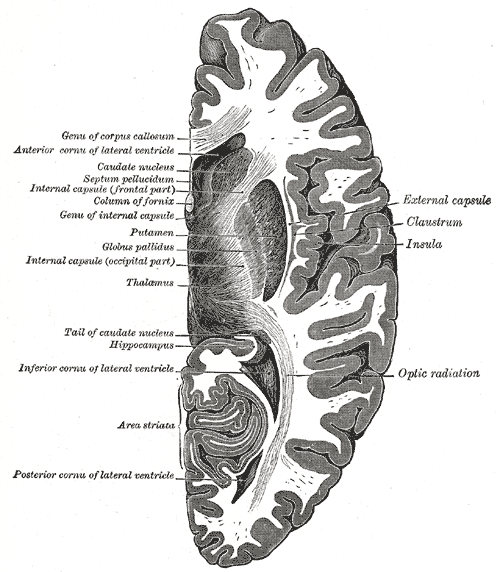
The hippocampus is the "flash drive" of the human brain and is often associated with memory consolidation and decision-making, but it is far more complex in structure and function than a flash drive. The hippocampus is a convex elevation of gray matter tissue within the parahippocampal gyrus inside the inferior temporal horn of the lateral ventricle. One can describe it more holistically as a curved and recurved sheet of the cortex that folds into the temporal lobe's medial surface. The hippocampus has three distinct zones: the dentate gyrus, the hippocampus proper, and the subiculum. The subiculum is positioned between the hippocampus proper and entorhinal and other cortices. The parahippocampal gyrus and cingulate sulci are located on the medial surface of the hemisphere, forming a C-shaped ring. The medial temporal lobe cortex includes major subdivisions, such as the hippocampus and the entorhinal cortex. This five-centimeter-long hippocampus (from the anterior end at the amygdala to the posterior end near the splenium of the corpus callosum) divides into a head, body, and tail.[1] the head is expanded and bears two or three shallow grooves called pes hippocampi. The head of the hippocampus is part of the posterior half of the triangular uncus and is separated inferiorly from the parahippocampal gyrus by the uncal sulcus. The alveus, which is the surface of the hippocampus, is covered by the ependymal inside the ventricular cavity.
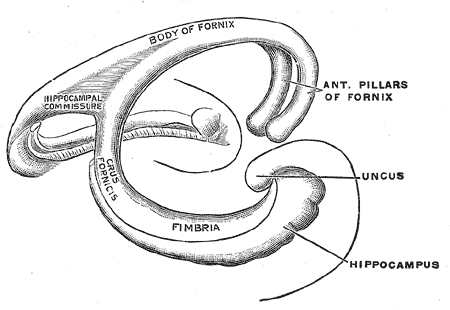
The fornix, which is the main outflow bundle out of the hippocampus, wraps around the thalamus, where it then becomes separated by the choroidal fissure and the choroid plexus. The hippocampus contains parts like the fimbria, crus, body, and column—the fimbria forms where alveus fibers converge along the medial portion of the lateral ventricle's inferior horn. The white matter of the fimbria separates to form a crux of the ipsilateral fornix at a point beyond the splenium of the corpus callosum. The Cornu Ammonis (CA) is a seahorse-like or ram's horn-like structure that describes the different layers of the hippocampus. There are four hippocampal subfields CA1, CA2, CA3, and CA4. CA3 and CA2 border the hilus of the dentate gyrus on either side. CA3 is the largest in the hippocampus and receives fibers from the dentate granule cells on their proximal dendrites[2]. The pyramidal cell layer is about ten cells thick.