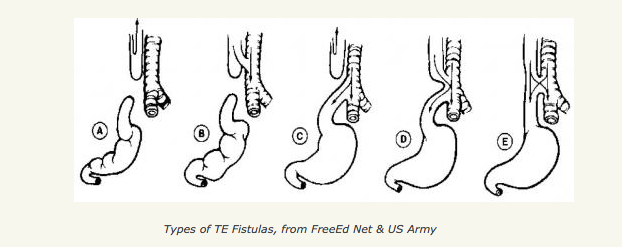
[1]
Crisera CA,Grau JB,Maldonado TS,Kadison AS,Longaker MT,Gittes GK, Defective epithelial-mesenchymal interactions dictate the organogenesis of tracheoesophageal fistula. Pediatric surgery international. 2000
[PubMed PMID: 10898225]
[2]
Ngan ES,Kim KH,Hui CC, Sonic Hedgehog Signaling and VACTERL Association. Molecular syndromology. 2013 Feb
[PubMed PMID: 23653575]
[3]
Diez-Pardo JA,Baoquan Q,Navarro C,Tovar JA, A new rodent experimental model of esophageal atresia and tracheoesophageal fistula: preliminary report. Journal of pediatric surgery. 1996 Apr
[PubMed PMID: 8801299]
[4]
Spilde T,Bhatia A,Ostlie D,Marosky J,Holcomb G 3rd,Snyder C,Gittes G, A role for sonic hedgehog signaling in the pathogenesis of human tracheoesophageal fistula. Journal of pediatric surgery. 2003 Mar
[PubMed PMID: 12632368]
[5]
Robert E,Mutchinick O,Mastroiacovo P,Knudsen LB,Daltveit AK,Castilla EE,Lancaster P,Källén B,Cocchi G, An international collaborative study of the epidemiology of esophageal atresia or stenosis. Reproductive toxicology (Elmsford, N.Y.). 1993 Sep-Oct
[PubMed PMID: 8274816]
[6]
Harper PS, Some pioneers of European human genetics. European journal of human genetics : EJHG. 2017 May 10
[PubMed PMID: 28488680]
[7]
Felix JF,de Jong EM,Torfs CP,de Klein A,Rottier RJ,Tibboel D, Genetic and environmental factors in the etiology of esophageal atresia and/or tracheoesophageal fistula: an overview of the current concepts. Birth defects research. Part A, Clinical and molecular teratology. 2009 Sep
[PubMed PMID: 19452513]
Level 3 (low-level) evidence
[9]
Depaepe A,Dolk H,Lechat MF, The epidemiology of tracheo-oesophageal fistula and oesophageal atresia in Europe. EUROCAT Working Group. Archives of disease in childhood. 1993 Jun
[PubMed PMID: 8333763]
[10]
Torfs CP,Curry CJ,Bateson TF, Population-based study of tracheoesophageal fistula and esophageal atresia. Teratology. 1995 Oct
[PubMed PMID: 8838292]
[11]
WATERSTON DJ,BONHAM-CARTER RE,ABERDEEN E, Congenital tracheo-oesophageal fistula in association with oesophageal atresia. Lancet (London, England). 1963 Jul 13
[PubMed PMID: 13999160]
[12]
Spitz L,Kiely EM,Morecroft JA,Drake DP, Oesophageal atresia: at-risk groups for the 1990s. Journal of pediatric surgery. 1994 Jun
[PubMed PMID: 8078005]
[13]
Choudhury SR,Ashcraft KW,Sharp RJ,Murphy JP,Snyder CL,Sigalet DL, Survival of patients with esophageal atresia: influence of birth weight, cardiac anomaly, and late respiratory complications. Journal of pediatric surgery. 1999 Jan
[PubMed PMID: 10022146]
[14]
Goyal A,Jones MO,Couriel JM,Losty PD, Oesophageal atresia and tracheo-oesophageal fistula. Archives of disease in childhood. Fetal and neonatal edition. 2006 Sep
[PubMed PMID: 16923940]
[15]
Keckler SJ,St Peter SD,Valusek PA,Tsao K,Snyder CL,Holcomb GW 3rd,Ostlie DJ, VACTERL anomalies in patients with esophageal atresia: an updated delineation of the spectrum and review of the literature. Pediatric surgery international. 2007 Apr
[PubMed PMID: 17377826]
[16]
Touloukian RJ,Keller MS, High proximal pouch esophageal atresia with vertebral, rib, and sternal anomalies: an additional component to the VATER association. Journal of pediatric surgery. 1988 Jan
[PubMed PMID: 3351734]
[17]
Pretorius DH,Drose JA,Dennis MA,Manchester DK,Manco-Johnson ML, Tracheoesophageal fistula in utero. Twenty-two cases. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1987 Sep
[PubMed PMID: 3312629]
Level 3 (low-level) evidence
[18]
Karnak I,Senocak ME,Hiçsönmez A,Büyükpamukçu N, The diagnosis and treatment of H-type tracheoesophageal fistula. Journal of pediatric surgery. 1997 Dec
[PubMed PMID: 9433996]
[19]
Zacharias J,Genc O,Goldstraw P, Congenital tracheoesophageal fistulas presenting in adults: presentation of two cases and a synopsis of the literature. The Journal of thoracic and cardiovascular surgery. 2004 Aug
[PubMed PMID: 15282472]
Level 3 (low-level) evidence
[20]
Stringer MD,McKenna KM,Goldstein RB,Filly RA,Adzick NS,Harrison MR, Prenatal diagnosis of esophageal atresia. Journal of pediatric surgery. 1995 Sep
[PubMed PMID: 8523220]
[21]
Sparey C,Jawaheer G,Barrett AM,Robson SC, Esophageal atresia in the Northern Region Congenital Anomaly Survey, 1985-1997: prenatal diagnosis and outcome. American journal of obstetrics and gynecology. 2000 Feb
[PubMed PMID: 10694347]
Level 3 (low-level) evidence
[22]
Okamoto T,Takamizawa S,Arai H,Bitoh Y,Nakao M,Yokoi A,Nishijima E, Esophageal atresia: prognostic classification revisited. Surgery. 2009 Jun
[PubMed PMID: 19486772]
[23]
Laffan EE,Daneman A,Ein SH,Kerrigan D,Manson DE, Tracheoesophageal fistula without esophageal atresia: are pull-back tube esophagograms needed for diagnosis? Pediatric radiology. 2006 Nov
[PubMed PMID: 16967270]
[24]
Nagata K,Kamio Y,Ichikawa T,Kadokura M,Kitami A,Endo S,Inoue H,Kudo SE, Congenital tracheoesophageal fistula successfully diagnosed by CT esophagography. World journal of gastroenterology. 2006 Mar 7
[PubMed PMID: 16552825]
[25]
Haight C, Congenital Atresia of the Esophagus With Tracheoesophageal Fistula : Reconstruction of Esophageal Continuity by Primary Anastomosis. Annals of surgery. 1944 Oct
[PubMed PMID: 17858517]
[26]
Babu R,Pierro A,Spitz L,Drake DP,Kiely EM, The management of oesophageal atresia in neonates with right-sided aortic arch. Journal of pediatric surgery. 2000 Jan
[PubMed PMID: 10646774]
[27]
Engum SA,Grosfeld JL,West KW,Rescorla FJ,Scherer LR 3rd, Analysis of morbidity and mortality in 227 cases of esophageal atresia and/or tracheoesophageal fistula over two decades. Archives of surgery (Chicago, Ill. : 1960). 1995 May
[PubMed PMID: 7748088]
Level 3 (low-level) evidence
[28]
Ein SH,Stringer DA,Stephens CA,Shandling B,Simpson J,Filler RM, Recurrent tracheoesophageal fistulas seventeen-year review. Journal of pediatric surgery. 1983 Aug
[PubMed PMID: 6620086]
[29]
Hagberg S,Rubenson A,Sillén U,Werkmäster K, Management of long-gap esophagus: experience with end-to-end anastomosis under maximal tension. Progress in pediatric surgery. 1986
[PubMed PMID: 3081964]
[30]
Rothenberg SS, Thoracoscopic management of non-type C esophageal atresia and tracheoesophageal atresia. Journal of pediatric surgery. 2017 Oct 12
[PubMed PMID: 29092772]
[31]
Rothenberg SS, Thoracoscopic repair of esophageal atresia and tracheo-esophageal fistula. Seminars in pediatric surgery. 2005 Feb
[PubMed PMID: 15770583]
[32]
Holcomb GW 3rd,Rothenberg SS,Bax KM,Martinez-Ferro M,Albanese CT,Ostlie DJ,van Der Zee DC,Yeung CK, Thoracoscopic repair of esophageal atresia and tracheoesophageal fistula: a multi-institutional analysis. Annals of surgery. 2005 Sep
[PubMed PMID: 16135928]
[33]
Puri P,Ninan GK,Blake NS,Fitzgerald RJ,Guiney EJ,O'Donnell B, Delayed primary anastomosis for esophageal atresia: 18 months' to 11 years' follow-up. Journal of pediatric surgery. 1992 Aug
[PubMed PMID: 1403548]
[34]
Foker JE,Kendall TC,Catton K,Khan KM, A flexible approach to achieve a true primary repair for all infants with esophageal atresia. Seminars in pediatric surgery. 2005 Feb
[PubMed PMID: 15770584]
[35]
Goyal A,Potter F,Losty PD, Transillumination of H-type tracheoesophageal fistula using flexible miniature bronchoscopy: an innovative technique for operative localization. Journal of pediatric surgery. 2005 Jun
[PubMed PMID: 15991163]
[36]
Bhatnagar V,Lal R,Sriniwas M,Agarwala S,Mitra DK, Endoscopic treatment of tracheoesophageal fistula using electrocautery and the Nd:YAG laser. Journal of pediatric surgery. 1999 Mar
[PubMed PMID: 10211655]
[37]
Meier JD,Sulman CG,Almond PS,Holinger LD, Endoscopic management of recurrent congenital tracheoesophageal fistula: a review of techniques and results. International journal of pediatric otorhinolaryngology. 2007 May
[PubMed PMID: 17408757]
[38]
Holland AJ,Ford WD,Guerin RL, Median sternotomy and use of a pedicled sternocleidomastoid muscle flap in the management of recurrent tracheoesophageal fistula. Journal of pediatric surgery. 1998 Apr
[PubMed PMID: 9574775]
[39]
Hoelzer DJ,Luft JD, Successful long-term endoscopic closure of a recurrent tracheoesophageal fistula with fibrin glue in a child. International journal of pediatric otorhinolaryngology. 1999 May 25
[PubMed PMID: 10402124]
[40]
Gdanietz K,Wiesner B,Krause I,Mau H,Jung FJ, [Tissue-adhesive for sealing of oesophagotracheal fistulae in children (author's transl)]. Zeitschrift fur Erkrankungen der Atmungsorgane. 1974
[PubMed PMID: 4460420]
[41]
HOLINGER PH,BROWN WT,MAURIZI DG, ENDOSCOPIC ASPECTS OF POST-SURGICAL MANAGEMENT OF CONGENITAL ESOPHAGEAL ATRESIA AND TRACHEOESOPHAGEAL FISTULA. The Journal of thoracic and cardiovascular surgery. 1965 Jan
[PubMed PMID: 14261873]
[42]
Krishnan U,Mousa H,Dall'Oglio L,Homaira N,Rosen R,Faure C,Gottrand F, ESPGHAN-NASPGHAN Guidelines for the Evaluation and Treatment of Gastrointestinal and Nutritional Complications in Children With Esophageal Atresia-Tracheoesophageal Fistula. Journal of pediatric gastroenterology and nutrition. 2016 Nov
[PubMed PMID: 27579697]
[43]
Teich S,Barton DP,Ginn-Pease ME,King DR, Prognostic classification for esophageal atresia and tracheoesophageal fistula: Waterston versus Montreal. Journal of pediatric surgery. 1997 Jul
[PubMed PMID: 9247237]
[44]
Konkin DE,O'hali WA,Webber EM,Blair GK, Outcomes in esophageal atresia and tracheoesophageal fistula. Journal of pediatric surgery. 2003 Dec
[PubMed PMID: 14666453]
[45]
Upadhyaya VD,Gangopadhyaya AN,Gupta DK,Sharma SP,Kumar V,Pandey A,Upadhyaya AD, Prognosis of congenital tracheoesophageal fistula with esophageal atresia on the basis of gap length. Pediatric surgery international. 2007 Aug
[PubMed PMID: 17579871]
[46]
David TJ,O'Callaghan SE, Oesophageal atresia in the South West of England. Journal of medical genetics. 1975 Mar
[PubMed PMID: 1121014]
[47]
Little DC,Rescorla FJ,Grosfeld JL,West KW,Scherer LR,Engum SA, Long-term analysis of children with esophageal atresia and tracheoesophageal fistula. Journal of pediatric surgery. 2003 Jun
[PubMed PMID: 12778380]
[48]
Chetcuti P,Phelan PD, Gastrointestinal morbidity and growth after repair of oesophageal atresia and tracheo-oesophageal fistula. Archives of disease in childhood. 1993 Feb
[PubMed PMID: 8481035]
[49]
Spitz L, Esophageal atresia and tracheoesophageal fistula in children. Current opinion in pediatrics. 1993 Jun
[PubMed PMID: 8374655]
Level 3 (low-level) evidence
[50]
Chetcuti P,Phelan PD, Respiratory morbidity after repair of oesophageal atresia and tracheo-oesophageal fistula. Archives of disease in childhood. 1993 Feb
[PubMed PMID: 8481036]
[51]
Chetcuti P,Phelan PD,Greenwood R, Lung function abnormalities in repaired oesophageal atresia and tracheo-oesophageal fistula. Thorax. 1992 Dec
[PubMed PMID: 1494766]
[52]
Antoniou D,Soutis M,Christopoulos-Geroulanos G, Anastomotic strictures following esophageal atresia repair: a 20-year experience with endoscopic balloon dilatation. Journal of pediatric gastroenterology and nutrition. 2010 Oct
[PubMed PMID: 20562719]
[53]
de Lagausie P, GER in oesophageal atresia: surgical options. Journal of pediatric gastroenterology and nutrition. 2011 May
[PubMed PMID: 21499041]
[54]
Somppi E,Tammela O,Ruuska T,Rahnasto J,Laitinen J,Turjanmaa V,Järnberg J, Outcome of patients operated on for esophageal atresia: 30 years' experience. Journal of pediatric surgery. 1998 Sep
[PubMed PMID: 9766349]
[55]
LeSouëf PN,Myers NA,Landau LI, Etiologic factors in long-term respiratory function abnormalities following esophageal atresia repair. Journal of pediatric surgery. 1987 Oct
[PubMed PMID: 3681622]
[56]
Jaureguizar E,Vazquez J,Murcia J,Diez Pardo JA, Morbid musculoskeletal sequelae of thoracotomy for tracheoesophageal fistula. Journal of pediatric surgery. 1985 Oct
[PubMed PMID: 4057018]
[57]
Cherup LL,Siewers RD,Futrell JW, Breast and pectoral muscle maldevelopment after anterolateral and posterolateral thoracotomies in children. The Annals of thoracic surgery. 1986 May
[PubMed PMID: 3707242]
[58]
Bianchi A,Sowande O,Alizai NK,Rampersad B, Aesthetics and lateral thoracotomy in the neonate. Journal of pediatric surgery. 1998 Dec
[PubMed PMID: 9869054]
[59]
Connor MJ,Springford LR,Kapetanakis VV,Giuliani S, Esophageal atresia and transitional care--step 1: a systematic review and meta-analysis of the literature to define the prevalence of chronic long-term problems. American journal of surgery. 2015 Apr
[PubMed PMID: 25605033]
Level 1 (high-level) evidence
[60]
Jayasekera CS,Desmond PV,Holmes JA,Kitson M,Taylor AC, Cluster of 4 cases of esophageal squamous cell cancer developing in adults with surgically corrected esophageal atresia--time for screening to start. Journal of pediatric surgery. 2012 Apr
[PubMed PMID: 22498376]
Level 3 (low-level) evidence
[61]
FEARON B, The endoscopic management of congenital atresia of the esophagus with or without tracheo-esophageal fistula and of congenital tracheo-esophageal fistula without atresia of the esophagus. The Annals of otology, rhinology, and laryngology. 1959 Dec
[PubMed PMID: 13821822]
[62]
DeBoer EM,Prager JD,Ruiz AG,Jensen EL,Deterding RR,Friedlander JA,Soden J, Multidisciplinary care of children with repaired esophageal atresia and tracheoesophageal fistula. Pediatric pulmonology. 2016 Jun
[PubMed PMID: 26422584]