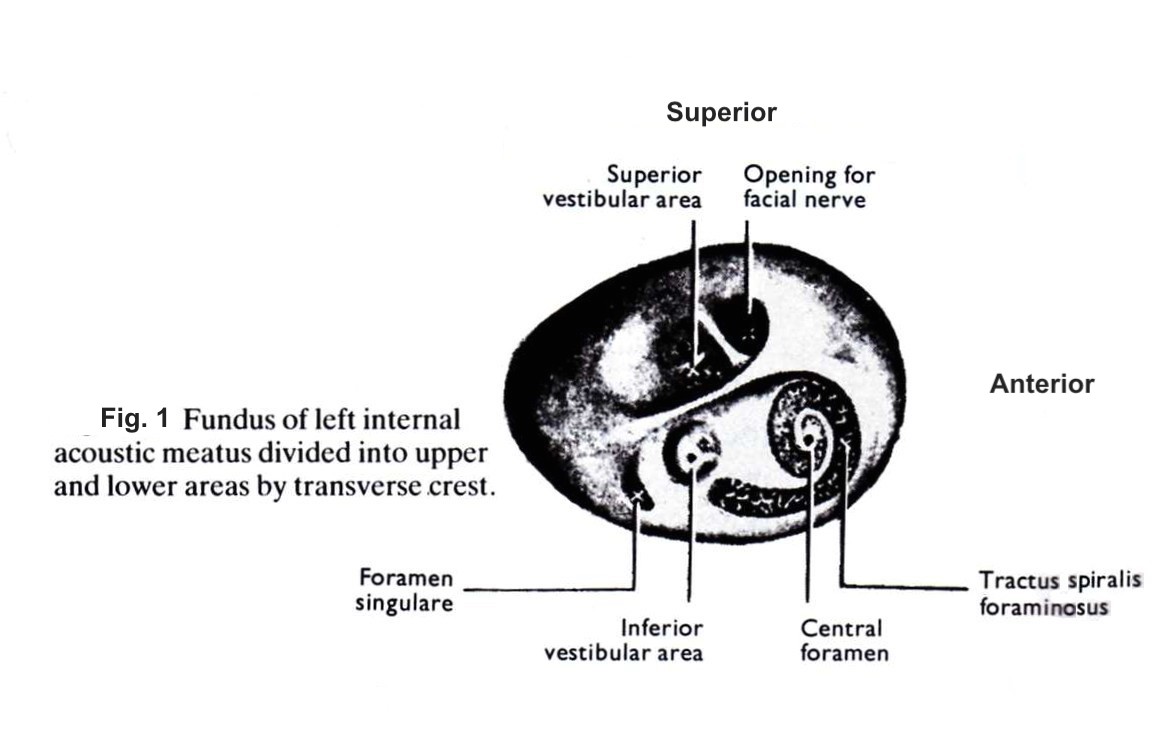
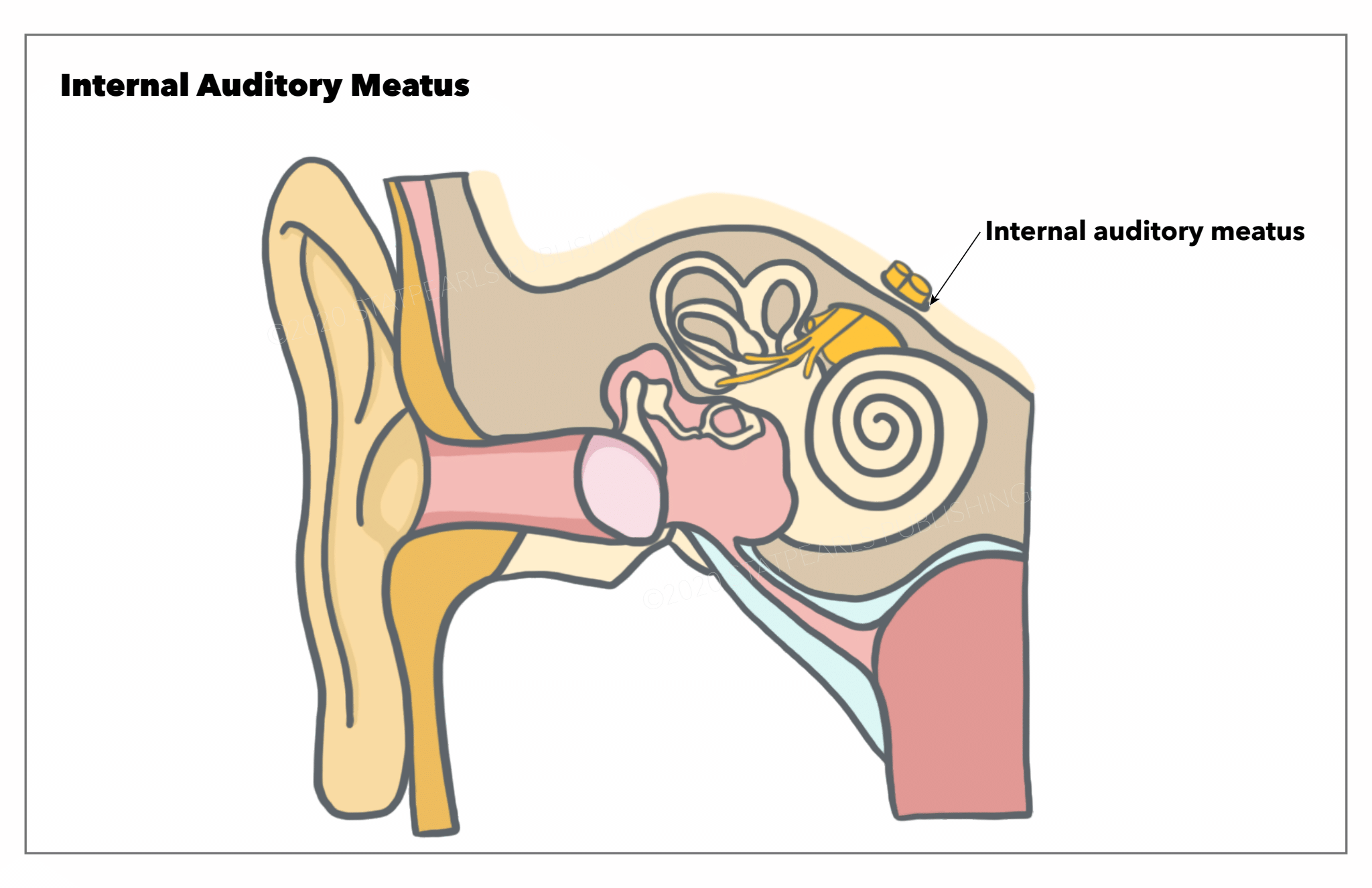
[1]
Fatterpekar GM, Mukherji SK, Lin Y, Alley JG, Stone JA, Castillo M. Normal canals at the fundus of the internal auditory canal: CT evaluation. Journal of computer assisted tomography. 1999 Sep-Oct:23(5):776-80
[PubMed PMID: 10524866]
[2]
Kozerska M,Skrzat J, Anatomy of the fundus of the internal acoustic meatus - micro-computed tomography study. Folia morphologica. 2015;
[PubMed PMID: 26339817]
[3]
Rubinstein D,Sandberg EJ,Cajade-Law AG, Anatomy of the facial and vestibulocochlear nerves in the internal auditory canal. AJNR. American journal of neuroradiology. 1996 Jun-Jul;
[PubMed PMID: 8791922]
[4]
Anniko M. Formation and maturation of the vestibular ganglion. ORL; journal for oto-rhino-laryngology and its related specialties. 1985:47(2):57-65
[PubMed PMID: 3982811]
[5]
Silverstein H, Norrell H, Smouha E, Haberkamp T. The singular canal: a valuable landmark in surgery of the internal auditory canal. Otolaryngology--head and neck surgery : official journal of American Academy of Otolaryngology-Head and Neck Surgery. 1988 Feb:98(2):138-43
[PubMed PMID: 3128756]
[6]
Rhoton AL Jr, Kobayashi S, Hollinshead WH. Nervus intermedius. Journal of neurosurgery. 1968 Dec:29(6):609-18
[PubMed PMID: 5708034]
[7]
Sakashita T,Sando I, Postnatal development of the internal auditory canal studied by computer-aided three-dimensional reconstruction and measurement. The Annals of otology, rhinology, and laryngology. 1995 Jun;
[PubMed PMID: 7771721]
[8]
Haidara A, Peltier J, Zunon-Kipre Y, N'da HA, Drogba L, Gars DL. Microsurgical Anatomy of the Labyrinthine Artery and Clinical Relevance. Turkish neurosurgery. 2015:25(4):539-43. doi: 10.5137/1019-5149.JTN.9136-13.0. Epub
[PubMed PMID: 26242329]
[9]
Khrais T, Romano G, Sanna M. Nerve origin of vestibular schwannoma: a prospective study. The Journal of laryngology and otology. 2008 Feb:122(2):128-31
[PubMed PMID: 18039415]
[10]
Brackmann DE. Acoustic neuroma: surgical approaches and complications. Annals of the Academy of Medicine, Singapore. 1991 Sep:20(5):674-9
[PubMed PMID: 1781654]
[11]
PERLMAN HB, KIMURA R, FERNANDEZ C. Experiments on temporary obstruction of the internal auditory artery. The Laryngoscope. 1959 Jun:69(6):591-613
[PubMed PMID: 13673604]
[12]
FERNANDEZ C. The effect of oxygen lack on cochlear potentials. The Annals of otology, rhinology, and laryngology. 1955 Dec:64(4):1193-203
[PubMed PMID: 13283493]
[13]
Ramly NA, Roslenda AR, Suraya A, Asma A. Vascular loop in the cerebellopontine angle causing pulsatile tinnitus and headache: a case report. EXCLI journal. 2014:13():192-6
[PubMed PMID: 26417253]
Level 3 (low-level) evidence
[14]
Gultekin S,Celik H,Akpek S,Oner Y,Gumus T,Tokgoz N, Vascular loops at the cerebellopontine angle: is there a correlation with tinnitus? AJNR. American journal of neuroradiology. 2008 Oct;
[PubMed PMID: 18653684]
[15]
Okamura T, Kurokawa Y, Ikeda N, Abiko S, Ideguchi M, Watanabe K, Kido T. Microvascular decompression for cochlear symptoms. Journal of neurosurgery. 2000 Sep:93(3):421-6
[PubMed PMID: 10969939]
[16]
Brackmann DE, Kesser BW, Day JD. Microvascular decompression of the vestibulocochlear nerve for disabling positional vertigo: the House Ear Clinic experience. Otology & neurotology : official publication of the American Otological Society, American Neurotology Society [and] European Academy of Otology and Neurotology. 2001 Nov:22(6):882-7
[PubMed PMID: 11698813]
[17]
Ryu H,Uemura K,Yokoyama T,Nozue M, Indications and results of neurovascular decompression of the eighth cranial nerve for vertigo, tinnitus and hearing disturbances. Advances in oto-rhino-laryngology. 1988;
[PubMed PMID: 3213742]
Level 3 (low-level) evidence
[18]
Gacek RR. Singular neurectomy update. The Annals of otology, rhinology, and laryngology. 1982 Sep-Oct:91(5 Pt 1):469-73
[PubMed PMID: 7137783]
[19]
Gacek RR, Gacek MR. Singular neurectomy in the management of paroxysmal positional vertigo. Otolaryngologic clinics of North America. 1994 Apr:27(2):363-79
[PubMed PMID: 8022615]
[21]
Silverstein H, Jackson LE. Vestibular nerve section. Otolaryngologic clinics of North America. 2002 Jun:35(3):655-73
[PubMed PMID: 12486846]