Continuing Education Activity
Primary breast cancer recurrence to the chest wall is a common. Hyperthermia to the chest wall recurrence, in conjunction with other available treatment modalities, can have a favorable impact on prognosis and outcomes. This activity reviews the evaluation and treatment of breast cancer that has recurred in the chest, specifically highlighting the utility of hyperthermia and its role in conjunction with other available cancer modalities including surgery, chemotherapy and radiation therapy, in improving prognosis and outcomes. This activity highlights the role of the interprofessional team in caring for affected patients.
Objectives:
- Describe the evaluation of breast cancer recurrence to the chest wall.
- Outline currently available treatment modalities for breast cancer recurrence to the chest wall.
- Explain the utility of hyperthermia in conjunction with surgery, chemotherapy and radiotherapy for the treatment of breast cancer recurrence to the chest wall.
- Review interprofessional team strategies for improving care coordination and communication to advance care for patients with breast cancer recurrence to the chest wall and improve outcomes.
Introduction
Current recommendations for treatment of local breast cancer recurrence include surgery, chemotherapy, hormone therapy, radiation alone, or radiation with hyperthermia. Tumor-directed hyperthermia (also called thermal therapy) is when the body or cancer tissue is heated to high temperatures, ranging from 40 to 43 C. When combined with radiation, hyperthermia plays a pivotal role in improving local control for breast patients with breast or chest wall recurrences that are chemo-refractory, not surgically resectable, and have had prior radiation. Hyperthermia helps to enhance the effects of radiation-induced, DNA damage by blocking DNA repair in cancer cells and by increasing oxygenation of tumors, needed for RT-induced free radical production to damage cancer cells. The considerable advantage of hyperthermia is the ability to combine it with lower doses of radiation therapy, leading to more effective outcomes with fewer side effects [1]. Hyperthermia is a specialized treatment that is only offered at select centers throughout the United States and around the world, so it is important for doctors to know more about this therapy so that they can help their patients get access. The purpose of this article is to review treatment options for local breast cancer recurrences, with a special focus on the role of hyperthermia.
Hyperthermia Mechanisms of Action
The applicability of hyperthermia in cancer treatment stems from the cytotoxic effects of elevated temperatures on tumor cells. Studies have shown temperatures between 41 to 44 C to be non-toxic to normal cells, but show toxicity in tumor cells [1]. Several cellular alterations induced by extreme temperatures [1] include
- Increased cellular permeability increasing the influx of Ca2+, an ion involved in the apoptotic signaling cascade, and increasing cellular permeability to drug delivery
- Disassembly of the cytoskeleton, enlarged tumor pores, enabling easier drug delivery
- Alteration of the mitotic spindles, centrosome organization, and protein denaturation which results in the formation of multinucleated non-clonogenic cells
- Alterations of the integrin-cytoskeleton network with concomitant cell shape change, anoikis, and programmed cell death
- Induction of DNA double-strand breaks due to the denaturation and dysfunction of heat-labile repair proteins such as DNA polymerases
- Precipitation of denatured proteins onto nuclear chromatin structures, generating a barrier which prevents repair enzymes from reaching damage sites
- Induction of apoptosis through generation of reactive oxygen species and increased expression of the pro-apoptotic BAX gene
Different Hyperthermia Modalities
Energies used to heat tumors include microwaves (wavelengths ranging from 433 to 2450 MHz), radiofrequency (ranging from 100 KHz to 150 MHz), ultrasound, hot water perfusion (tubes, blankets), resistive wire implants, ferromagnetic seeds, nanoparticles, and infrared radiators. Hyperthermia is more commonly administered with electromagnetic applicators since they appear to yield better power disposition and temperature distribution [2]. The superficial applicators include a waveguide, spiral, and current sheet, and are positioned on tumor surfaces with a bolus.
Etiology
Risk factors for chest wall recurrence include primary tumor size, primary stage, and lymph node involvement. In a study of 1031 patients by Katz et al., authors found that patients with tumors greater than or equal to 4 cm or at least 4 involved nodes experience local recurrence rates more than 20% [3]. The Early Breast Cancer Trialists' Collaborative Group reported a 10-year risk of locoregional recurrence without distant metastases as the initial event after mastectomy of 20.3% for patients with 1 to 3 positive axillary lymph nodes, and of 32.1% for patients with 4 or more positive nodes (EBCTCG, 2014). Additional factors that increase the risk of chest wall recurrence include ages 40 and younger, gross multifocal/multicentric disease, and genetic factors [4]. Histopathological risk factors include positive margin status, presence of DCIS, extensive intraductal component, high grade, lymphovascular invasion, tumor oncogene and tumor suppressor gene expression (for example, p53 and HER2), and estrogen receptor status [5]. Additionally, the use of adjuvant chemoradiation therapy has been demonstrated to reduce the risk of chest wall failure [6].
Epidemiology
Chest wall recurrence of breast cancer portends a poor prognosis and is usually accompanied by or antecedent to distant metastases. Regardless of this association, aggressive treatment of local, regional recurrence may allow patients to have longer disease-free survival. The 10-year incidence of local chest wall recurrence after mastectomy ranges from 5% to 45% in patients with both early and late stage breast cancer and tends to occur within the first 5 years of treatment. This rate decreases to about 2% to 15% in patients who receive postoperative radiation therapy [7]. In addition to chest wall failure, 10% to 20% of patients with stage I to IIIA breast cancer will have additional regional recurrences (including axillary and other nodal) [5]. The rate of development of distant metastases after local, regional recurrence has been reported as 35% and 6% in patients after no radiation therapy and with radiation therapy, respectively [8]. Indeed, local recurrence is an independent prognostic indicator for distant metastases. In a study of 6792 patients, isolated local, regional recurrence was 18%, and only 33% of these patients remained free of distant metastases [4]. After recurrence, 10-year disease-free survival and overall survival rates range from 7% to 12% and 22% to 26%, respectively [4].
History and Physical
Evidence of chest wall recurrence includes new mass or lump, a new area of thickness along the mastectomy scar, chest wall pain, skin changes including ulceration, bleeding, lymphedema, swelling, redness, inflammation, pruritus, and brachial plexopathy [7]. Along with these local signs, chest wall tumors may be accompanied by signs and symptoms of distant metastases that include, but are not limited to, dyspnea and persistent cough (e.g., metastatic to the lung), bone pain, loss of appetite, weight loss, and neurological symptoms if metastatic to the brain.
Evaluation
When there is suspicion of a chest wall recurrence, a biopsy of the lesion should first be done to confirm the histology. The ER/PR and HER2 status should be repeated for the recurrent tumor as there may be discordance between the primary and recurrent tumors, likely related to changes in the biology of the disease, tumor heterogeneity, divergent clonal subsets due to differential treatment effects, sampling errors or limited accuracy and reproducibility of assays [9], [10]. The patient should also undergo further imaging to make sure there are no signs of distant metastatic disease, such as a CT of the chest, abdomen, and pelvis. Other diagnostic scans could include PET/CT, MRI, or bone scans. Further recommendations on workup can be found in the NCCN guidelines (NCCN, 2018).
Treatment / Management
Prior to deciding on the treatment, the physician should discuss goals of care with the patient. If the patient's performance status is poor and they have widespread metastatic disease, then they may opt for less aggressive treatment. The following sections focus on patients who have decided to pursue locally aggressive measures, often with the intent to cure. Aggressive palliation can also be valuable in improving quality of life.
The management of patients with chest wall recurrence includes surgical resection where feasible, radiation therapy, chemotherapy, hormone therapy if ER/PR positive, and hyperthermia in conjunction with radiation therapy. Physicians should communicate with all patients in a multi-disciplinary fashion to determine the best treatment approach, as these patients likely will need a multi-modality approach to achieve the best outcomes.
Differential Diagnosis
- Multiple organ dysfunction syndromes
Surgical Oncology
In patients with surgically resectable disease, chest wall resection has been shown to have favorable results. In their study of full thickness chest wall resection for recurrent breast cancer, Toi et al. reported a 5-year survival rate of 47% [11]. Petrella et al. reported similar results in their 40 patients with full thickness chest wall resection, including a 5-year overall survival (OS) and disease-free survival (DFS) of 68.5% and 46%, respectively [12]. The authors reported age and synchronous distant metastases as factors adversely impacting overall survival. Several other studies have reported similar 5-year OS ranging from 35% to 62% [13], [14], [15]. Notably, chemotherapy was not given to any of the patients in these studies before resection.
If the patient is not initially resectable, chemotherapy, radiation, or the combination of chemoradiation may be used to attempt to shrink the tumor so that it is resectable. If the patient still cannot undergo surgery, then they likely will have less favorable outcomes. Notably, it has been shown that gross disease at the time of radiation therapy is associated with a lower 5-year local control rate (63%) and survival rate (34%) compared to patients without residual disease after resection and systemic therapy (81% and 62%, respectively) [16]. This underscores the utility of complete resection or systemic treatment to shrink the tumor, where feasible.
Radiation Oncology
Radiation Alone
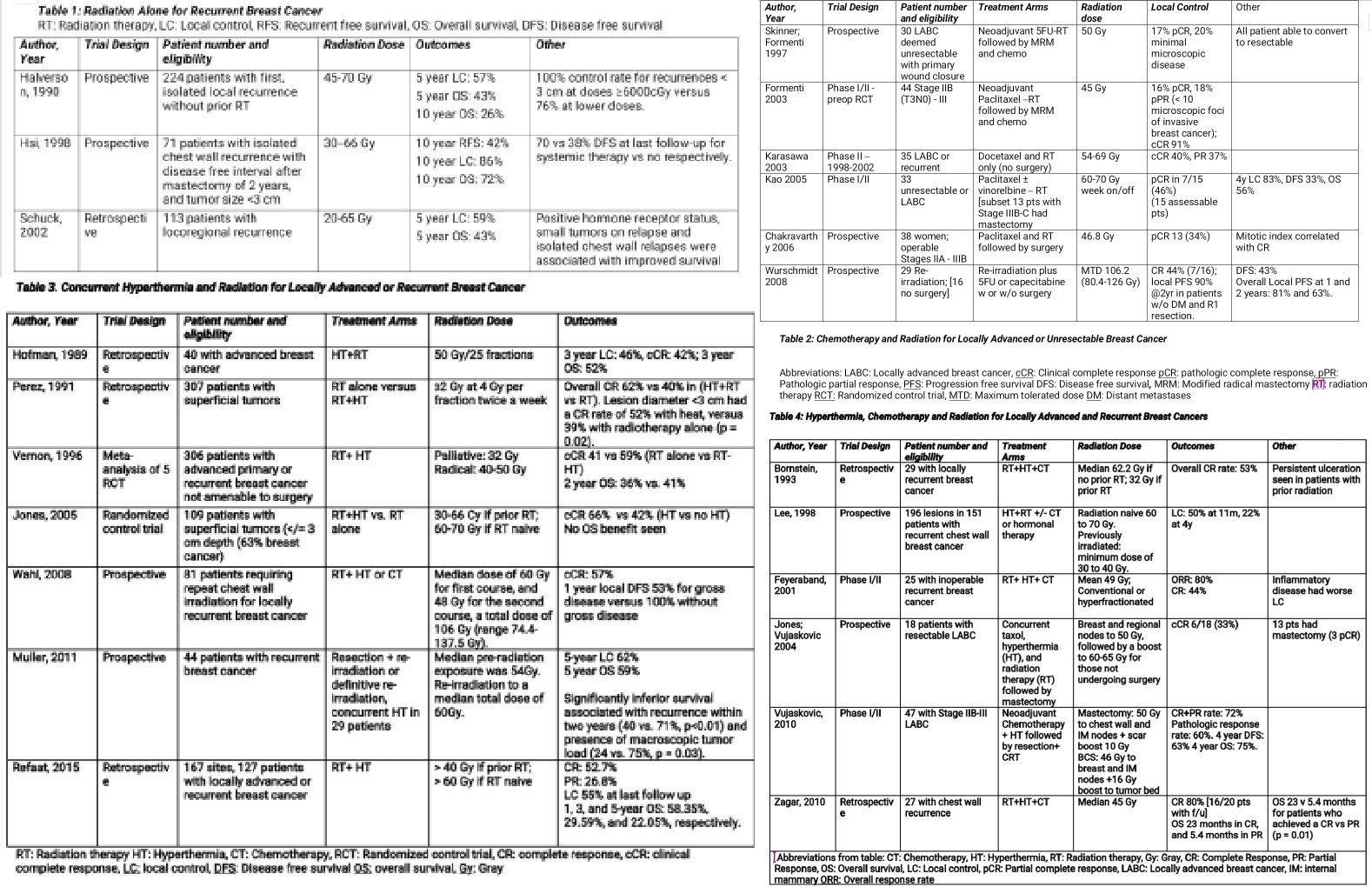
In the context of patients with chest wall recurrence and no prior radiation therapy, NCCN guidelines recommend definitive doses of chest wall and nodal irradiation. Adjuvant radiation has been shown to improve outcomes in multiple trials described in Table 1, where overall survival rates ranging from 57% to 86% have been reported. In Halverson’s study of 224 previously non-irradiated patients with isolated local-regional recurrence, improved outcomes were associated with treatment with larger radiation fields (entire chest wall), higher doses (greater than or equal to 6000 Gy), isolated recurrences, and smaller recurrent tumor size (less than 3 cm). The addition of prophylactic supraclavicular fossa radiation also improved supraclavicular failure rates (16% versus 6%, p < 0.05) [17]
Radiation and Chemotherapy
There are at least 6 small prospective trials with 29 to 44 patients, that reported that neoadjuvant or concurrent chemotherapy and radiation in either the locally advanced setting or recurrent setting, can result in a 16% to 47% complete pathologic response rate, as described in Table 2. Additionally, there is an ongoing phase II trial studying the impact of pembrolizumab, an anti-PD (Programmed Cell Death)-1 antibody, in combination with carboplatin compared to carboplatin alone for unresectable chest wall disease (NCT03393858).
Radiation with Hyperthermia
The addition of hyperthermia to radiation therapy has been shown to achieve complete response rates ranging from 55% to 66%, much higher than the 16% to 47% seen with chemoRT, as shown in Table 3. In breast cancer patients who develop unresectable and chemorefractory breast or chest wall recurrences, the combination of radiation and hyperthermia may offer the most robust local response, which may also translate to improved overall survival. Hyperthermia combined with radiation permits the advantage of lower radiation doses due to its radiosensitizing properties [7]; this is especially important in women who have received prior radiation therapy to the breast or chest wall. The ability to safely and effectively administer repeat radiation therapy is more difficult due to the toxicity associated with high cumulative radiation doses, as well as a lower likelihood of controlling a tumor that was originally resistant to prior definitive doses of radiation. It has additionally been shown that tumor size, minimum tumor temperature and radiation dose are important factors associated with the amount of tumor response to concurrent radiation and hyperthermia [18].
In a meta-analysis of 5 randomized control trials (1988 through 1991), 306 patients with unresectable advanced primary or recurrent breast cancer received either RT alone versus RT and hyperthermia and achieved a complete response rate of 41% versus 59%, respectively. The greatest benefit was observed in patients with recurrent lesions in previously irradiated areas, where the dose of radiation was lowered due to prior radiation treatment and concern of toxicity to nearby normal tissues, thus showing that the synergy of hyperthermia helped the lower doses of radiation reach a higher therapeutic ratio leading to local control. Even more, the addition of hyperthermia achieved a more prolonged complete response rate versus radiation alone [19].
A more recent randomized trial of 109 patients all treated with chemotherapy, plus RT and HT or RT alone, was able to achieve a 66% CR rate in the dual therapy group versus only 42% for RT alone. Local control was also more durable with the addition of HT (48% versus 25% at death and last follow up) [20]. Similar to seen in the Vernon study, this trial also found that previously irradiated patients benefited the most from the addition of hyperthermia: 23.5% in the no-HT arm versus 68.2% in the HT arm.
Wahl and colleagues analyzed clinical outcomes in 81 patients who underwent re-irradiation to the breast or chest wall. The median dose of the first course 60 Gy and 48 Gy for the second course, a total dose of 106 Gy (range 74.4 to 137.5 Gy). Patients additionally received concurrent hyperthermia, and/or chemotherapy. The authors found an overall complete response rate of 57% (67% with concurrent hyperthermia, and 39% without), a 1-year disease free survival of 53% for gross disease versus 100% without gross disease (P<0.001). Four patients developed late grade 3 or 4 toxicities, and no treatment related deaths occurred [21].
In another study by Muller et al on 44 patients with recurrent breast cancer, repeat macroscopically complete resection and re-irradiation to a total median dose of 54 Gy, plus hyperthermia (69% of patients), resulted in an estimated 5 year overall survival of 59%, a 5 year local control rate of 62% and no higher late toxicity greater than grade 3 was observed [22].
Hyperthermia, Chemotherapy, and Radiation (Triple Therapy)
Small, retrospective, and phase I/II studies have shown the beneficial outcomes of this triple therapy with local complete response rates ranging from 44% to 80%, as shown in Table 4. Acute toxicities associated with triple therapy have been reported as persistent ulceration seen in patients with prior radiation, blisters, second and third-degree burns, infection, and as expected with chemotherapy, bone marrow toxicity [18], [23], [19].
Lee et al. performed a prospective study from 1977 to 1990, on 151 patients with 196 subcutaneous/superficial lesions (179 measurable, 17 microscopic). The patients were treated with superficial 915-MHz microwave hyperthermia and irradiation. In patients without prior radiation, the radiation field encompassed the ipsilateral chest wall and supraclavicular area. Elective areas were treated to 46 to 50 Gy, followed by a boost to areas of gross disease, giving a total dose of 60 to 70 Gy. For previously radiated patients, the investigators extended the fields where normal tissue tolerance permitted, with a margin of 2 to 3 cm around all gross tumors, to a minimum dose of 30 to 40 Gy. The hyperthermia was delivered immediately after radiation, once or twice a week, separated by at least 72 hours, to a tumor temperature of at least 43 C. At the time of maximum tumor regression, there was a 63% CR rate, 19% PR rate, and 16% stable disease. There was a 50% LC rate at last follow up (median follow up 11 months), and 2 and 3 year LC control rates of 42% and 33%, respectively [24].
Vujoskovic et al. evaluated the efficacy of neoadjuvant liposomal doxorubicin (30 to 75 mg/m), paclitaxel (100 to 175 mg/m), and hyperthermia, followed by resection and radiation therapy and then 8 cycles of CMF (cyclophosphamide, methotrexate, 5-fluorouracil) chemotherapy, in 47 patients with stage IIB-III locally advanced breast cancer. The authors found a combined (partial plus complete) clinical response of 72% and pathological response of 60% (4 patients achieved a pathologically complete response) 4-year DFS and OS were 63% and 75%, respectively [25].
Hyperthermia and Immunotherapy
There is growing interest in the use of hyperthermia as an adjunct to immunotherapy in cancer treatment. Tumor cell release of heat shock proteins in response to hyperthermia allows the proteins to be endocytosed by dendritic cells, increasing cell surface expression of MHC class II molecules, and augmenting dendritic cell priming against those tumor antigens. Tumors treated with hyperthermia have been shown to exhibit improved dendritic cell priming and enhancement of systemic immunity to tumor cells. In one study by Takeda et al., the addition of hyperthermia to immunotherapy increased clinical benefit (CR, PR, and stable disease for more than 6 months) and the effective rate of immunotherapy from 7.7% to 26.0% [26].
Medical Oncology
Hormone Therapy
According to NCCN guidelines, premenopausal woman with recurrences that are estrogen receptor (ER) positive, and who were not previously exposed to hormone therapy, can be treated with selective modulators alone or ovarian suppression/ablation plus endocrine therapy. The Swiss Group for Clinical research compared tamoxifen versus observation after excision and radiation of isolated postmastectomy locoregional recurrence of breast cancer in 167 women (79% postmenopausal), Tamoxifen improved DFS from 2.7 to 6.5 years (p = .053), mainly due to the reduction of further local relapses. The investigators also reported tamoxifen led to an increase in DFS from 33% to 61% (p = 0.006) in postmenopausal woman [27]. The results of 4 clinical trials randomizing 506 premenopausal women with advanced breast cancer to luteinizing hormone-releasing hormone (LHRH) alone versus LHRH combined with tamoxifen found a significant survival benefit (HR = .78, p = .02) and progression-free survival (PFS) benefit (HR = .7, p = .0003) favoring combined therapy, at a median follow up of 6.8 years [28].
Current recommendations for recurrent disease in postmenopausal women include nonsteroidal aromatase inhibitors, steroidal aromatase inhibitors, ER modulators, ER down regulators, progestin, androgens, and high-dose estrogen (NCCN, 2018). In patients resistant to endocrine therapy or with ER-negative tumors, the addition of everolimus, an mTOR signal transduction pathway inhibitor, showed a complete or partial response rate of 61.1% when combined with tamoxifen versus 42.1% with tamoxifen alone, along with improvements in time to progression (median of 4.5 months in tamoxifen alone versus 8.5 months in combined therapy [29]. As the benefits of endocrine therapy outweigh their relatively low toxicity, and further false negative determinations of ER/PR status are not unusual, the NCCN panel recommends consideration of a trial of endocrine therapy for patients with disease characterized as ER-negative, localized to bone or soft tissue only, or with asymptomatic visceral disease (NCCN, 2018).
Chemotherapy
If the patient is deemed a chemotherapy candidate, and their tumor is chemo-responsive, then they may benefit from improved locoregional control. The Chemotherapy for Isolated Locoregional Recurrence of Breast Cancer (CALOR) Randomised Trial, a study of 162 patients with isolated local regional recurrence treated with adjuvant chemotherapy after surgery and radiation, showed that chemotherapy improved 5-year DFS and OS compared to no chemotherapy: 69% versus 57% (p = .046) and 88% versus 76% (p = .02), respectively. The choice of chemotherapy was left to the discretion of the investigators, but at least 2 cytotoxic drugs were recommended [30]. Patients should discuss the latest chemotherapy options or clinical trials for recurrent disease.
Treatment Planning for Radiation and Hyperthermia
Hyperthermia
Local external hyperthermia is most beneficial when delivered twice a week after radiation therapy, for about 1 hour and at least 48 hours apart to relatively small, superficial tumors (less than or equal to 3 cm up to 5 to 6 cm) where the goal is to achieve tumor temperatures between 41 to 44 C.
Ideally, attempts should be made to maximize tumor temperatures while limiting surrounding tissue temperatures to no greater than 44 C. Temperature parameters to be monitored during treatment include initial tumor and tissue temperatures, maximum, minimum, and average temperatures.
Radiation Dose
The radiation dosing and fractionation will depend on prior treatment, with lower total doses and sometimes twice-daily RT given in the re-irradiation setting. Doses can range between 30 and 66 Gy for previously irradiated patients, and 60 to 70 Gy for previously un-irradiated patients. Consensus has not been reached on safe cumulative doses, and patients should be counseled on the possibility of grade 3 or 4 late toxicities, including fibrosis, tissue necrosis, pain, infection, and lymphedema.
Radiation Fields
The radiation target volume, beam energy and angles, and dose utilized will depend on goals of care, the extent of disease, extent of prior radiation exposure, time between initial radiation treatment and retreatment, skin condition and appearance at the time of retreatment, and doses to the adjacent organs and tissue at risk. Electron or photon fields may be used in patients previously irradiated. In general, if the patient had prior radiation to the whole breast or chest wall, then the whole field is not usually re-treated. The radiation target focuses on the recurrent gross disease plus a margin for microscopic disease and set-up error, with the normal tissue constraints often liming the total dose. The radiation oncologist must customize each radiation plan per patient’s anatomy and goals of care.
The following fields have been used in previously irradiated patients:
- Jones et al. used electron fields encompassing all gross disease with a minimum 2 to 3 cm margin [20]
- Kapp et al. used fields encompassing the entire region of recurrence, with cone down boosts delivered using electron beams in the scar area with 2 to 3 cm margins in all directions [31].
Local breast cancer recurrences often herald a poor prognosis. Despite its association with metastatic breast cancer, aggressive treatment of isolated locoregional recurrence may still improve survival. In addition to current treatment standards, including surgical resection, chemotherapy, hormone therapy, and radiation therapy, the addition of hyperthermia has been found to improve outcomes in these patients. While achieving a complete response rate is a worthwhile goal, palliation of symptoms is also invaluable for patients. The utility of concurrent hyperthermia and radiation therapy is apparent; however, hyperthermia is not available at all centers. Physicians should seek out centers with hyperthermia to coordinate external beam radiation treatments for these select patients. There is also a critical need for innovative treatments to help improve outcomes for patients who recur despite previous breast cancer therapies.
Pearls and Other Issues
- Hyperthermia is often delivered twice a week within an hour after daily radiation, and it enhances the effects of radiation-induced DNA damage by blocking DNA repair in cancer cells, increasing blood flow thereby increasing oxygenation and free oxygen radicals, as well as several other direct effects on tumor cell death.
- The use of radiation and hyperthermia to tumor temperatures ranging from 41 to 44 C has been shown to improve local control in locally recurrent breast cancer patients.
- The combination of radiation with chemotherapy and radiation therapy can be safely administered to tissue as superficial as the chest wall, with minimal toxicity.
- Hyperthermia may not be available at all centers. However, it is imperative to try to coordinate hyperthermia treatments with nearby hyperthermia centers to provide the best chance for local control for locally recurrent breast cancer patients.
Enhancing Healthcare Team Outcomes
Patients with metastatic disease to the chest wall are best managed by an interprofessional team that also includes oncology nurses. Before deciding on hyperthermia, the patient functional status must be established. This treatment is not curative and is only palliative. Patients too ill should not undergo this treatment as it offers no benefit. In addition, patients with widespread metastases are not ideal candidates. The outcomes for most patients with metastatic disease to the chest wall are poor, irrespective of therapy. Hence, palliative care nurses should be involved early in their care.