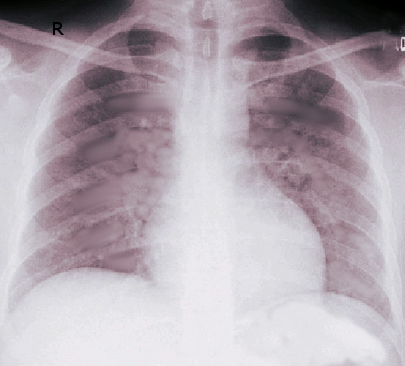
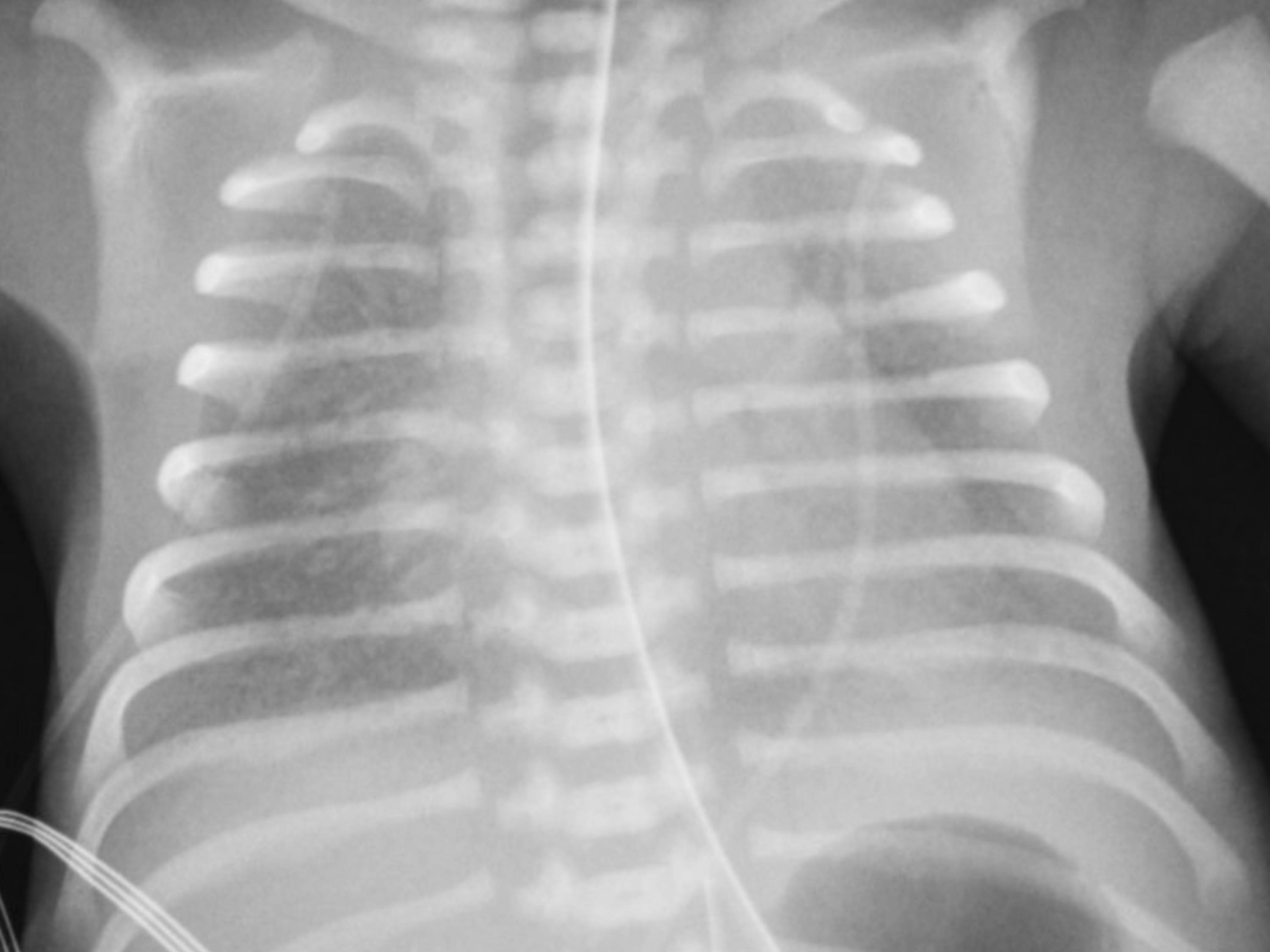
[1]
Gajic O, Dabbagh O, Park PK, Adesanya A, Chang SY, Hou P, Anderson H 3rd, Hoth JJ, Mikkelsen ME, Gentile NT, Gong MN, Talmor D, Bajwa E, Watkins TR, Festic E, Yilmaz M, Iscimen R, Kaufman DA, Esper AM, Sadikot R, Douglas I, Sevransky J, Malinchoc M, U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS). Early identification of patients at risk of acute lung injury: evaluation of lung injury prediction score in a multicenter cohort study. American journal of respiratory and critical care medicine. 2011 Feb 15:183(4):462-70. doi: 10.1164/rccm.201004-0549OC. Epub 2010 Aug 27
[PubMed PMID: 20802164]
[2]
Wang Y, Zhang L, Xi X, Zhou JX, China Critical Care Sepsis Trial (CCCST) Workgroup. The Association Between Etiologies and Mortality in Acute Respiratory Distress Syndrome: A Multicenter Observational Cohort Study. Frontiers in medicine. 2021:8():739596. doi: 10.3389/fmed.2021.739596. Epub 2021 Oct 18
[PubMed PMID: 34733862]
[3]
Zambon M, Vincent JL. Mortality rates for patients with acute lung injury/ARDS have decreased over time. Chest. 2008 May:133(5):1120-7. doi: 10.1378/chest.07-2134. Epub 2008 Feb 8
[PubMed PMID: 18263687]
[4]
Shrestha GS, Khanal S, Sharma S, Nepal G. COVID-19: Current Understanding of Pathophysiology. Journal of Nepal Health Research Council. 2020 Nov 13:18(3):351-359. doi: 10.33314/jnhrc.v18i3.3028. Epub 2020 Nov 13
[PubMed PMID: 33210623]
Level 3 (low-level) evidence
[5]
Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld G, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, LUNG SAFE Investigators, ESICM Trials Group. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016 Feb 23:315(8):788-800. doi: 10.1001/jama.2016.0291. Epub
[PubMed PMID: 26903337]
[6]
Sedhai YR, Yuan M, Ketcham SW, Co I, Claar DD, McSparron JI, Prescott HC, Sjoding MW. Validating Measures of Disease Severity in Acute Respiratory Distress Syndrome. Annals of the American Thoracic Society. 2021 Jul:18(7):1211-1218. doi: 10.1513/AnnalsATS.202007-772OC. Epub
[PubMed PMID: 33347379]
[7]
Sharma NS, Lal CV, Li JD, Lou XY, Viera L, Abdallah T, King RW, Sethi J, Kanagarajah P, Restrepo-Jaramillo R, Sales-Conniff A, Wei S, Jackson PL, Blalock JE, Gaggar A, Xu X. The neutrophil chemoattractant peptide proline-glycine-proline is associated with acute respiratory distress syndrome. American journal of physiology. Lung cellular and molecular physiology. 2018 Nov 1:315(5):L653-L661. doi: 10.1152/ajplung.00308.2017. Epub 2018 Aug 9
[PubMed PMID: 30091378]
[8]
Huang D, Ma H, Xiao Z, Blaivas M, Chen Y, Wen J, Guo W, Liang J, Liao X, Wang Z, Li H, Li J, Chao Y, Wang XT, Wu Y, Qin T, Su K, Wang S, Tan N. Diagnostic value of cardiopulmonary ultrasound in elderly patients with acute respiratory distress syndrome. BMC pulmonary medicine. 2018 Aug 13:18(1):136. doi: 10.1186/s12890-018-0666-9. Epub 2018 Aug 13
[PubMed PMID: 30103730]
Level 2 (mid-level) evidence
[9]
Vieillard-Baron A, Schmitt JM, Augarde R, Fellahi JL, Prin S, Page B, Beauchet A, Jardin F. Acute cor pulmonale in acute respiratory distress syndrome submitted to protective ventilation: incidence, clinical implications, and prognosis. Critical care medicine. 2001 Aug:29(8):1551-5
[PubMed PMID: 11505125]
[10]
Chen WL, Lin WT, Kung SC, Lai CC, Chao CM. The Value of Oxygenation Saturation Index in Predicting the Outcomes of Patients with Acute Respiratory Distress Syndrome. Journal of clinical medicine. 2018 Aug 8:7(8):. doi: 10.3390/jcm7080205. Epub 2018 Aug 8
[PubMed PMID: 30096809]
[11]
Rawal G, Yadav S, Kumar R. Acute Respiratory Distress Syndrome: An Update and Review. Journal of translational internal medicine. 2018 Jun:6(2):74-77. doi: 10.1515/jtim-2016-0012. Epub 2018 Jun 26
[PubMed PMID: 29984201]
[12]
Cherian SV, Kumar A, Akasapu K, Ashton RW, Aparnath M, Malhotra A. Salvage therapies for refractory hypoxemia in ARDS. Respiratory medicine. 2018 Aug:141():150-158. doi: 10.1016/j.rmed.2018.06.030. Epub 2018 Jul 3
[PubMed PMID: 30053961]
[13]
Guérin C, Reignier J, Richard JC, Beuret P, Gacouin A, Boulain T, Mercier E, Badet M, Mercat A, Baudin O, Clavel M, Chatellier D, Jaber S, Rosselli S, Mancebo J, Sirodot M, Hilbert G, Bengler C, Richecoeur J, Gainnier M, Bayle F, Bourdin G, Leray V, Girard R, Baboi L, Ayzac L, PROSEVA Study Group. Prone positioning in severe acute respiratory distress syndrome. The New England journal of medicine. 2013 Jun 6:368(23):2159-68. doi: 10.1056/NEJMoa1214103. Epub 2013 May 20
[PubMed PMID: 23688302]
[14]
National Heart, Lung, and Blood Institute PETAL Clinical Trials Network, Moss M, Huang DT, Brower RG, Ferguson ND, Ginde AA, Gong MN, Grissom CK, Gundel S, Hayden D, Hite RD, Hou PC, Hough CL, Iwashyna TJ, Khan A, Liu KD, Talmor D, Thompson BT, Ulysse CA, Yealy DM, Angus DC. Early Neuromuscular Blockade in the Acute Respiratory Distress Syndrome. The New England journal of medicine. 2019 May 23:380(21):1997-2008. doi: 10.1056/NEJMoa1901686. Epub 2019 May 19
[PubMed PMID: 31112383]
[15]
National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network, Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, Connors AF Jr, Hite RD, Harabin AL. Comparison of two fluid-management strategies in acute lung injury. The New England journal of medicine. 2006 Jun 15:354(24):2564-75
[PubMed PMID: 16714767]
[16]
Brodie D, Bacchetta M. Extracorporeal membrane oxygenation for ARDS in adults. The New England journal of medicine. 2011 Nov 17:365(20):1905-14. doi: 10.1056/NEJMct1103720. Epub
[PubMed PMID: 22087681]
[17]
Combes A, Hajage D, Capellier G, Demoule A, Lavoué S, Guervilly C, Da Silva D, Zafrani L, Tirot P, Veber B, Maury E, Levy B, Cohen Y, Richard C, Kalfon P, Bouadma L, Mehdaoui H, Beduneau G, Lebreton G, Brochard L, Ferguson ND, Fan E, Slutsky AS, Brodie D, Mercat A, EOLIA Trial Group, REVA, and ECMONet. Extracorporeal Membrane Oxygenation for Severe Acute Respiratory Distress Syndrome. The New England journal of medicine. 2018 May 24:378(21):1965-1975. doi: 10.1056/NEJMoa1800385. Epub
[PubMed PMID: 29791822]
[18]
Yang P, Formanek P, Scaglione S, Afshar M. Risk factors and outcomes of acute respiratory distress syndrome in critically ill patients with cirrhosis. Hepatology research : the official journal of the Japan Society of Hepatology. 2019 Mar:49(3):335-343. doi: 10.1111/hepr.13240. Epub 2018 Aug 29
[PubMed PMID: 30084205]
[19]
Annane D, Pastores SM, Rochwerg B, Arlt W, Balk RA, Beishuizen A, Briegel J, Carcillo J, Christ-Crain M, Cooper MS, Marik PE, Umberto Meduri G, Olsen KM, Rodgers S, Russell JA, Van den Berghe G. Correction to: Guidelines for the diagnosis and management of critical illness-related corticosteroid insufficiency (CIRCI) in critically ill patients (Part I): Society of Critical Care Medicine (SCCM) and European Society of Intensive Care Medicine (ESICM) 2017. Intensive care medicine. 2018 Mar:44(3):401-402. doi: 10.1007/s00134-018-5071-6. Epub
[PubMed PMID: 29476199]
[20]
Rodrigo C, Leonardi-Bee J, Nguyen-Van-Tam J, Lim WS. Corticosteroids as adjunctive therapy in the treatment of influenza. The Cochrane database of systematic reviews. 2016 Mar 7:3():CD010406. doi: 10.1002/14651858.CD010406.pub2. Epub 2016 Mar 7
[PubMed PMID: 26950335]
Level 1 (high-level) evidence
[21]
Anand R, McAuley DF, Blackwood B, Yap C, ONeill B, Connolly B, Borthwick M, Shyamsundar M, Warburton J, Meenen DV, Paulus F, Schultz MJ, Dark P, Bradley JM. Mucoactive agents for acute respiratory failure in the critically ill: a systematic review and meta-analysis. Thorax. 2020 Aug:75(8):623-631. doi: 10.1136/thoraxjnl-2019-214355. Epub 2020 Jun 8
[PubMed PMID: 32513777]
Level 1 (high-level) evidence
[22]
Gadre SK, Duggal A, Mireles-Cabodevila E, Krishnan S, Wang XF, Zell K, Guzman J. Acute respiratory failure requiring mechanical ventilation in severe chronic obstructive pulmonary disease (COPD). Medicine. 2018 Apr:97(17):e0487. doi: 10.1097/MD.0000000000010487. Epub
[PubMed PMID: 29703009]
[23]
Chiumello D, Coppola S, Froio S, Gotti M. What's Next After ARDS: Long-Term Outcomes. Respiratory care. 2016 May:61(5):689-99. doi: 10.4187/respcare.04644. Epub
[PubMed PMID: 27121623]
[24]
Villar J, Schultz MJ, Kacmarek RM. The LUNG SAFE: a biased presentation of the prevalence of ARDS! Critical care (London, England). 2016 Apr 25:20(1):108. doi: 10.1186/s13054-016-1273-x. Epub 2016 Apr 25
[PubMed PMID: 27109238]
[25]
Bos LD, Cremer OL, Ong DS, Caser EB, Barbas CS, Villar J, Kacmarek RM, Schultz MJ, MARS consortium. External validation confirms the legitimacy of a new clinical classification of ARDS for predicting outcome. Intensive care medicine. 2015 Nov:41(11):2004-5. doi: 10.1007/s00134-015-3992-x. Epub 2015 Jul 23
[PubMed PMID: 26202043]
Level 3 (low-level) evidence