Introduction
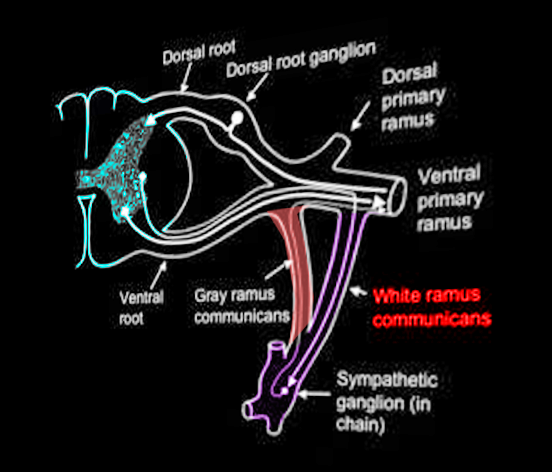
The white ramus communicans (pl. white rami communicantes), which is also known as the white communicating branch or the white communicating ramus, contains preganglionic fibers of the sympathetic system. The white ramus communicans is a structure that anteriorly connects the spinal nerve to the sympathetic trunk. All preganglionic sympathetic neurons traverse through a white ramus communicans. Though sympathetic neurons emerge from the spinal cord through the ventral root to enter a white ramus, their courses after that can vary in four different ways. Sympathetic neurons follow the pattern of passing through the white ramus as preganglionic neurons, synapsing onto a ganglion, and then innervating their respective organs as postganglionic neurons. Ultimately, these sympathetic fibers target blood vessels, sweat glands, arrector pili muscles, and visceral organs to elicit a "fight or flight" physiological response.
Structure and Function
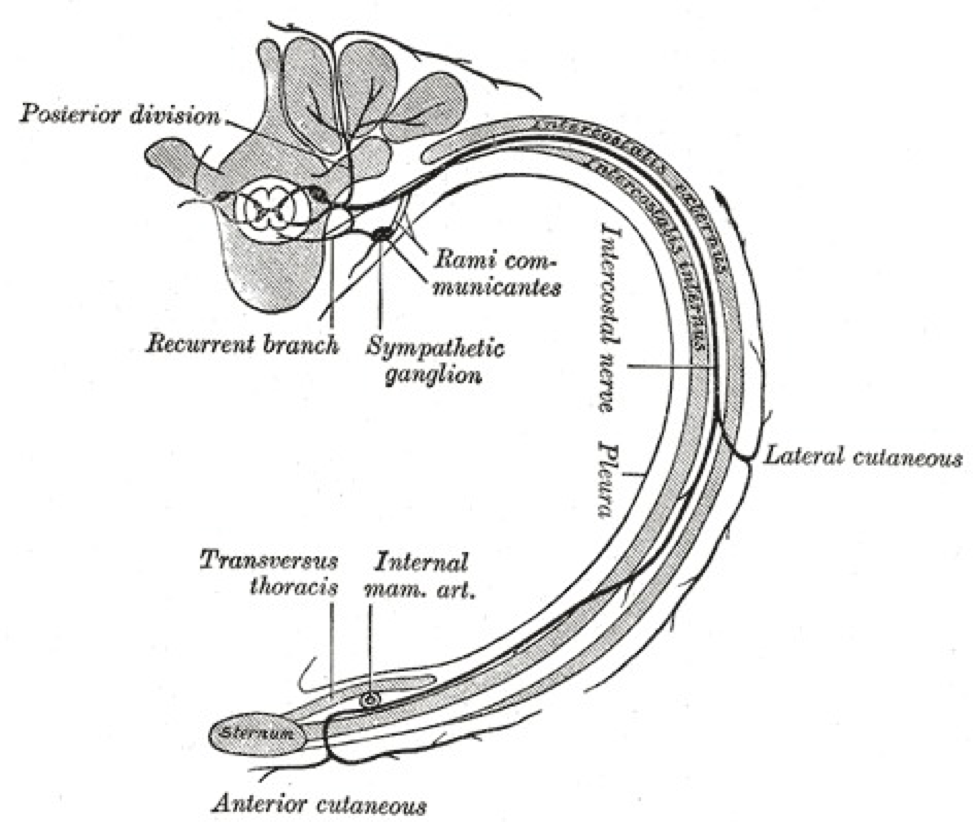
White rami communicantes connect preganglionic sympathetic neurons of the spinal cord to the sympathetic trunk. The sympathetic trunk contains a sympathetic ganglion for each corresponding vertebral level. White rami communicantes bring organization to the sympathetic outflow of nerves. Although white rami communicantes contain both myelinated and unmyelinated axons, they contain a higher density of myelinated axons. Hence, the term “white” in its name. White rami are lateral to the gray rami, their post-ganglionic unmyelinated sympathetic counterpart.
White rami communicantes function ipsilaterally and appear on both sides of the spinal column. As part of the sympathetic system, white rami communicantes originate from the thoracolumbar (T1 to L2) vertebral outflows. Above the T1 and below the L2 vertebral outflows, white rami are absent; only gray rami exist in these regions. In the thoracic portion (T1 to T12), the thoracic nerve divides into the anterior and ventral rami of the thoracic nerve. White rami communicantes originate from the anterior rami of the thoracic nerve, which are also called the intercostal nerves. In the lumbar regions where white rami are found (L1 to L2), the white rami communicantes originate directly from the lumbar nerves.
Nerves of white rami have distinct courses. Neurons that traverse through the white rami originate from the intermediolateral cell column (lateral horn of the spinal cord). The intermediolateral cell column spans from T1-L2.[1] From the intermediolateral cell column, sympathetic neurons exit the spinal cord through the ventral root and merge at the spinal nerve with other neurons. Only sympathetic neurons then enter a white ramus communicans, which branches anteriorly from the spinal nerve onto the sympathetic trunk. The white rami communicantes provide a path for preganglionic sympathetic neurons of the spinal nerve to enter the sympathetic trunk. From here, neurons can take one of four courses:
- Ascend or descend the sympathetic trunk. Without synapsing onto its corresponding sympathetic ganglion, axons can ascend or descend the sympathetic trunk before synapsing into a ganglion of a different level. Postganglionic sympathetic nerves can then travel to the spinal nerve via gray ramus communicans.
- Enter the cardiopulmonary splanchnic nerve. In T2 to T4 vertebral levels, neurons of the white rami communicantes synapse at their corresponding sympathetic ganglion and enter the thoracic, cardiac branches. These postganglionic neurons eventually innervate the heart, lungs, and trachea.
- Enter the abdominopelvic splanchnic nerve. In T5 to L2, some sympathetic neurons can course through the white rami communicantes and sympathetic trunk without synapsing. In T5 to T12, neurons travel through a splanchnic nerve (the greater, the lesser, or the least thoracic splanchnic nerve) and synapse at a peripheral ganglion. In L1 to L2, neurons travel through the lumbar splanchnic nerve before synapsing at a peripheral ganglion. The postganglionic sympathetic neurons can then proceed to innervate visceral organs, such as the stomach, liver, intestines, kidney, adrenal glands, pancreas, bladder, gonads, and genitals.
Postganglionic entrance into a spinal nerve typically involves the gray rami communicantes. Sympathetic innervation to the heart and other visceral organs involves splanchnic nerves that directly emerge from the sympathetic ganglion.
The sympathetic preganglionic neurons supported by the white rami communicantes eventually target blood vessels, sweat glands, and arrector pili muscles. The sympathetic nerves of the splanchnic routes lead to the autonomic control of the heart and visceral organs. The white rami communicantes provide a structure that separates and guides preganglionic sympathetic neurons into the sympathetic trunk and its ganglia.[2][3][4]
Embryology
White rami communicantes derive from trunk neural crest cells. These neural crest cells migrate ventrally from the dorsal portion of the neural tube and give rise to neuroblasts. Some of the sympathetic neuroblasts synapse with fibers of the spinal cord to form the white rami communicantes and other portions of the sympathetic nervous system.[5]
Blood Supply and Lymphatics
The white rami communicantes are usually close to the intercostal lymph nodes and intercostal lymph vessels.
Nerves
In addition to holding preganglionic sympathetic outflows of the spinal cord, such as efferent outflows, the T5 to T9 white rami communicantes can also contain general afferent (sensory) visceral inflows. These general afferent visceral fibers classify as neither sympathetic nor parasympathetic; however, visceral afferent fibers tend to associate anatomically with sympathetic efferent fibers. As a result, signals from sensory receptors of visceral organs also travel through the peripheral ganglion, sympathetic trunk, and the white rami communicantes. After traveling through the white rami communicantes and the spinal nerve, these visceral afferent fibers then enter the spinal cord via the dorsal root of the spinal cord. Afferent visceral nerves often synapse onto the preganglionic sympathetic neurons located in the intermediolateral cell column.[6]
Muscles
White rami communicantes hold fibers that eventually innervate cardiac tissue, arrector pili muscles, and smooth muscle of visceral organs and vasculature.
Physiologic Variants
Physiological variations, especially those located in the upper thoracic regions (T2 to T5), are abundant for white rami communicantes:
- In the T1 level, the white rami communicantes of the spinal nerve can appear medial to its corresponding gray rami communicantes. Normally, white rami are lateral to gray rami.
- The horizontal distance from the sympathetic trunk to the distal point of a white ramus communicans can range from 2.5 to 28.5 millimeters.
- Sometimes T5 can also send postganglionic sympathetic neurons into the cardiothoracic nerve branches.
- In T2, T3, and T4 levels, it is possible for additional gray or white rami to extend to a different level ganglion of the sympathetic trunk. T2 experiences the most significant presence of ascending and descending rami.
- It is possible, in fact, quite frequent (85.7%), to observe bilateral asymmetry when comparing white and gray rami.
- When considering both gray and white rami communicantes, one of each communicating rami are expected to enter each ganglion. However, it is possible for a sympathetic ganglion in T2, T3, and T4 ganglion levels to have anywhere from 1 to 4 communicating rami.
- Occasionally, white rami might exist at the L3 level.[7]
Surgical Considerations
Sympathectomy and ramicotomy, the procedures of disrupting the sympathetic trunk and the communicating branches, can be conducted to attenuate the effects of conditions such as hyperhidrosis. Compared to sympathectomy, which has been considered the “gold standard” for the treatment of idiopathic hyperhidrosis, the procedure of ramicotomy requires a longer operation time. It can result in broader variability in the severity of compensatory sweating.[8]
Clinical Significance
Sympathectomy is the most common surgical treatment used to fix severe hyperhidrosis. Hyperhidrosis is a condition that results in excessive sweating in specific areas of the body, such as the face, hands, and armpits. Several studies have explored the effects of ramicotomy in treating hyperhidrosis. One of the main side effects of both sympathectomy and ramicotomy involves compensatory hyperhidrosis, which is the compensatory sweating in other large areas of the body. Compensatory sweating is typically the chief post-operative patient complaints.
Severe axillary hyperhidrosis, palmar hyperhidrosis, and craniofacial hyperhidrosis have received treatment with ramicotomy. In all types of hyperhidrosis, selective ramicotomy of the upper thoracic region has shown an improvement in the quality of life and reduced focal sweating. Some instances showed that ramicotomy often resulted in a lowered rate of compensatory sweating, while other cases displayed an increased rate of compensatory sweating. Post-operative compensatory hyperhidrosis due to ramicotomy for the treatment of severe hyperhidrosis can display a wide range of severity.[8][9][10][11]