Continuing Education Activity
The anterior spinal artery syndrome refers to ischemic infarction of the spinal cord resulting from direct occlusion of the anterior spinal artery, artery of Adamkiewicz, or generalized hypoperfusion. It is associated with many other disease conditions or treatment complications. Prompt diagnosis and treatment are necessary to avoid high morbidity-mortality, long-term disability related to anterior spinal artery syndrome. This activity reviews the evaluation and treatment of anterior spinal artery syndrome and highlights the role of the interprofessional team in evaluating and treating patients with anterior spinal artery syndrome.
Objectives:
- Identify the etiology of the anterior spinal artery syndrome.
- Summarize the pathophysiology of the anterior spinal artery syndrome.
- Outline and explain different treatment modalities for anterior spinal artery syndrome.
- Explain the importance of improving care coordination amongst the interprofessional team to enhance the delivery of care for patients with the anterior spinal artery syndrome.
Introduction
The anterior spinal artery supplies the anterior two-thirds of the spinal cord and runs along the entire length of the anterior surface of the spinal cord.[1] If the blood flow through the anterior spinal artery becomes occluded, the anterior two-thirds of the spinal cord will suffer an infarct, resulting in bilateral lower extremity paresis or paraplegia with loss of pain and temperature sensation. This clinical manifestation is known as anterior spinal artery syndrome (ASAS). There will be the preservation of proprioception, vibratory sense, and fine touch. The neurological deficits will manifest below the level of insult due to the anatomical distribution of the spinal cord tracts affected.[2][3]
The ASAS is a rare cause of spinal cord acute ischemic myelopathy but is the most common cause of spinal cord infarcts.[1][2][3] The neurological clinical deficit was first described in 1904 by Preobraschenki, while the pathoanatomical description was documented in 1909 by Spider.[2] ASAS is caused by any etiology that decreases or affects blood flow to the anterior spinal artery, most commonly, aortic surgery and atherosclerotic disease.[2] Prognosis is generally poor, as there is no acute management available for ASAS. Management consists of identifying the underlying etiology, treatment of symptoms, and prevention of complications.[2]
Etiology
ASAS occurs when occlusion/hypoperfusion occurs at the anterior spinal artery or any major radiculomedullary branches.[2][4] The artery of Adamkiweicz is the most common location for occlusion. The occlusion/hypoperfusion will produce ischemia in the vascular distribution of the anterior spinal artery.[1][2][5]
Many etiologies have correlations with occlusion/hypoperfusion of the anterior spinal artery.
- Aortic surgery (iatrogenic): most common cause of ASAS.[2][5] Some factors that may increase the risk for complications are hypotension, prolonged cross-clamping of the aorta, increased spinal cord pressure, and occlusion of radiculomedullary arteries. All of these factors may decrease blood flow through the anterior spinal artery and lead to ischemia. Other complications may involve direct surgical injury to major radiculomedullary arteries, such as the artery of Adamkiweicz.[5][6][7]
- Atherosclerotic disease: a common cause of ASAS. Development of a thrombus/emboli that occludes the anterior spinal artery leading to ASAS.[5][8]
- Thoraco-abdominal aortic disease (aortic dissection): dissection of the aorta causes occlusion of radiculomedullary arteries.[5]
- Cardiac arrest: absent blood flow to the anterior spinal artery
- Cardiac emboli: This is an infrequent cause of ASAS. This category includes infectious endocarditis, which may lead to septic emboli, and this may lead to an anterior spinal artery or major radiculomedullary arteries occlusion and thus the development of ASAS.[9]
- Vasculitis: a rare cause of ASAS. (ie. polyarteritis nodosa).[10]
- Degenerative spine disease: although a rare cause of ASAS, the literature describes patients with cervical spondylosis or thoracic disc herniation that present with ASAS. A large disc herniation can compress the anterior spinal artery and cause spinal cord infarction resulting in ASAS.[1][11]
- Vertebral fracture: anterior cord impingement by a fracture fragment.[3]
- Fibrocartilaginous embolic myelopathy: This condition is sometimes known as idiopathic transverse myelitis. Naiman et al. first described it in 1961.[12] Migration of nucleus pulposus material into vessels supplying the anterior spinal artery may result in embolic infarction of the spinal cord and hence, ASAS.[10][12][13]
- Shock: hypotension leads to ASAS.[4]
- Luetic aortitis: it was common during the syphilitic era, but rarely seen at present.[2]
- Minimally invasive spine surgery: rare iatrogenic cause. Percutaneous balloon kyphoplasty.[14][15]
- Arteriovenous malformations (AVM): spinal cord AVM.[16]
- Digital subtraction angiography: a rare cause of ASAS during diagnostic and embolization procedures. The overall incidence is 1.4 to 6.5%.[4]
- Sickle cell disease: thrombosis.[17]
- Other hypercoagulable diseases: thrombosis.[18][19]
- Cocaine use: vasospasm, vasculitis, and thrombosis.[20][21][22][23]
Epidemiology
ASAS is a rare condition; therefore, statistical studies evaluating the incidence and prevalence of this condition are scarce.[2][5] Approximately 5-8% of the cases of acute spinal myelopathies are due to vascular causes such as spinal cord infarctions.[3][10]
Spinal cord infarction accounts for 1.2% of all strokes. Spinal cord infarct has an incidence in the USA of 3.1 per 100,000 persons.[24] Studies with small groups have described spinal cord infarction in patients whose ages range from the 1st decade to the 10th decade, with a median age of presentation between 50-70 years old.[25] ASAS is the most common of all spinal cord infarctions described in some studies in up to 87.2% of the cases.[8][26]
Some risk factors associated with ASAS development include smoking, hypertension, diabetes mellitus, dyslipidemia, and family or patient history of ischemic cerebrovascular accidents.[2][5] There is no statistical evidence for a gender preference in ASAS; however, literature has reported that old aged females with ASAS have the worst prognosis.[5]
Pathophysiology
The anterior spinal artery (ASA), supplies the anterior two-thirds of the medulla/spinal cord. Two arteries branch from their respective vertebral arteries and form a single ASA at the foramen magnum level. The ASA runs as an uninterrupted artery located within the anterior median sulcus up to the conus medullaris.[5] Although it is a continuous artery, it is composed of multiple anastomotic networks, and it is also supplied by the anterior medullar arteries (or radiculomedullary arteries).[4] It varies in caliber along its course, smallest being at the thoracic level (making it most vulnerable to ischemia at this area) and largest at the lumbar level.[4]
The anterior spinal artery gives off small sulcal and penetrating arteries that enter the spinal cord's body at each level to supply blood to the anterior two-thirds of the spinal cord.[5] Each level of the spinal cord contains a radicular artery that enters through the intervertebral foramen, which primarily supplies the nerve root and dura. At some levels, they are enlarged and include embryonic connections with the ASA, making them radiculomedullary arteries.[4]
At cervical regions, the ASA connections from radiculomedullary arteries can be variable. Usually, the cut off is above the aortic arch, from either the vertebral or subclavian arteries. At thoracic and lumbar regions, the intercostal arteries give rise to the radiculomedullary arteries, supplying the ASA. They supply the spinal cord at and below their level of entry. The spinal cord has, on average, from 7 to 8 radiculomedullary arteries. The largest of all radiculomedullary arteries is called the great anterior radiculomedullary artery or artery of Adamkiwiecz.
The artery of Adamkiwiecz is the largest and most common radiculomedullary artery occluded and results in the ischemia of ASA and ASAS development. It supplies the lower two-thirds of the spinal cord. It originates from the intercostal artery most commonly from the left side (70% to 80%).[4] Its origin may vary. T9-T12 is the most common site of origin (75%), T5-T8 (15%), L1-L2 (10%).[3][4][5] On digital subtraction angiography, it is described as a straight vessel with a hairpin turn with subsequent communication with the anterior spinal artery coursing along the spine's midline.[4]
To understand the clinical presentation of ASAS, it is essential to know the spinal cord's anatomy to explain the pathophysiology behind this condition. The spinal cord subdivides into three portions. The posterior part of the cord contains the fasciculus gracilis and cuneatus responsible for proprioception, vibration sense, and fine touch. This area is supplied by the two posterior spinal arteries that run in the spinal cord's posterior lateral sulcus, and it is why these modalities are preserved in ASAS.[3][5][6]
The anterolateral portion of the cord contains the spinothalamic and spinocerebellar tracts responsible for transmitting pain and temperature sensation from extremities to the brain.[6] These portions are watershed areas in the spine, and the level of dysfunction will depend on the level of ASAS ischemic insult.[6] The level of sensory deficits may not be directly related to the exact injury level because spinothalamic tracts slope 2 or 3 levels upwards before crossing at the anterior commissure of the spinal cord.[3] The anteromedial portion contains the corticospinal and corticobulbar tracts responsible for transmitting motor function from the brain to extremities. They are completely affected, given that the anterior spinal artery supplies them.[5][6]
The lateral horns exist from the T1 level to the L2 level. They contain the cell bodies of the sympathetic nervous system. The anterior spinal artery is responsible for the vascular supply to these areas. If an infarction occurs at these levels, the sympathetic network is affected, for which patients may present with neurogenic bladder/bowel and sexual impairments.[5]
After the ASA flow becomes reduced or absent, several events at the cellular level occur. This disruption of blood flow starts a signaling cascade that results in neuronal destruction. It begins with the ischemic insult in which there is a decrease in blood flow and oxygen to the spinal cord. There is immediate activation of astrocytes and microglia, tissue edema/inflammation, disruption of the brain-blood-barrier, and neutrophil influx.[3] This disruption leads to ionic pump failure and anoxic depolarization, which leads to an increased concentration of intracellular calcium and glutamate. These, in part, produce increased activation of the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptors and N-methyl-D-aspartate receptor, alteration in the metabolic and mitochondrial activity, which ultimately produces cell death. The increased intracellular calcium produces free radical, elevations of nitric oxide production, and cytotoxic edema further contributing to cell death. A later reperfusion injury occurs during the subacute stage, where cell death occurs secondary to the inflammatory oxidative response with the activation of the apoptotic cascade.[3]
History and Physical
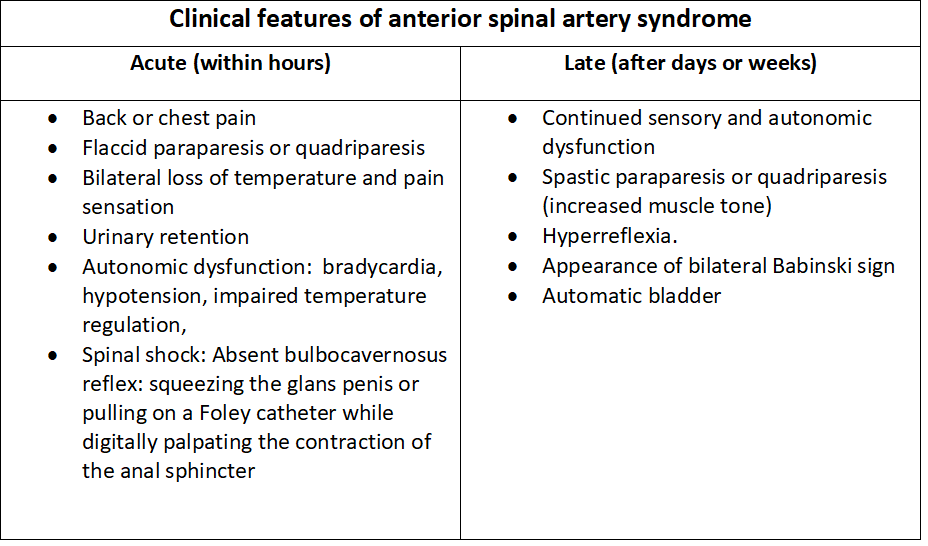
The development of ASAS is associated with an acute presentation after the time of insult. Usually, the first presenting symptom is acute back pain, whose location most of the time corresponds with the level of the injury in the spinal cord.[2][3] Below the spinal cord level of injury, there is loss/dysfunction of bilateral motor function, loss of pain and temperature sensation, but with preserved proprioception, vibratory sense, fine touch, and two-point discrimination.[1][2][3][5][6] The patient may present autonomic dysfunction findings such as bowel/bladder dysfunction, hypotension, and bradycardia if the lateral horns are affected.[3] If the injury is at a high cervical level, dysfunction of the phrenic nerve may lead to respiratory failure. Clinical findings are usually bilateral due to the spinal cord supply by the anterior spinal artery; however, a unilateral presentation has been described.[5] Since ASAS has no acute treatment, late signs and symptoms, including spasticity, hyperreflexia, neurogenic bladder, and sexual dysfunction, are usually present.
Acute clinical findings:
- Acute back pain at the level of injury
- Bilateral flaccid para/quadriparesis or plegia
- Loss of pain and temperature
- Preservation of proprioception, vibratory sense, fine touch, and two-point discrimination
- Autonomic dysfunction with hypotension, bradycardia, and impaired temperature regulation
- Respiratory failure
- Spinal shock
Late clinical findings (within days to weeks):
- Continued/permanent motor/sensory dysfunction
- Bilateral spastic para/quadriparesis or plegia
- Hyperreflexia
- Bilateral Babinski signs
- Neurogenic bladder/bowel
- Sexual dysfunction
Evaluation
The diagnosis of ASAS is usually suspected with a detailed history and the physical exam findings. Neuroimaging studies can help establish and confirm the diagnosis of ASAS.[1][2]
Magnetic resonance imaging (MRI) of the spine is the gold-standard study for the diagnosis of ASAS. Although results can be negative in the first 24 hours, it is usually done to confirm an ASAS diagnosis.[2] The hallmark finding is a hyperintensity at anterior horns in the T2-weighted image.[3][5] Findings suggesting ASAS are a thin "pencil-like" hyperintense region that extends vertically involving multiple spinal levels in the sagittal view and on the axial view two bright dots at each anterior horn described as "owl's eyes."[5] Other findings that suggest a spinal cord infarct are T1-weighted hypointensity at the area of injury, spinal cord expansion at the injury site due to early signs of inflammation/edema (diffusion-weighted images may help distinguish between ischemia and inflammation), and signs of vertebral body infarction which is not always present. Still, when it is, it increases specificity for cord infarction.[2][5] The MRI study helps to identify possible etiologies behind ASAS, such as conditions that may be compressing the spinal cord like herniated disc or tumor.[5]
Magnetic resonance angiography may help for further delineation and visualization of spinal vascular pathology.[3] Computed tomographic angiography helps in the identification of the anterior spinal artery. It also gives adequate visualization of the bone anatomy, however, exposes the patient to radiation doses and nephrotoxic agents.[1][5] It may help to rule out an aortic or vertebral artery dissection.[3] Digital subtraction angiography can also be used but is not the study of choice.[1] It is an ideal imaging technique for diagnosing spinal vascular lesions.[1] It helps to identify aortic or vertebral dissection.[5]
Other studies and tests are useful to find the underlying etiology of ASAS.[3][5] Lumbar puncture with cerebrospinal fluid (CSF) analysis helps rule out multiple sclerosis, infection, or inflammatory disease. An echocardiogram may help to rule out sources of embolism, such as infective endocarditis. Blood and urine laboratory studies can rule out infection (borreliosis, Lyme disease, syphilis, human immunodeficiency virus, herpes virus), hypercoagulable disorders, atherosclerotic disease, inflammatory disorders, rheumatological diseases, or drug-related.[3][5]
Treatment / Management
There is no effective treatment to stop or revert ASAS. The use of high dose corticosteroids for patients with ASAS is ineffective, and few reports have shown mild improvement, while most of them show no improvement.[1][6] In cases of atherosclerotic or embolic disease as the underlying etiology, patients treated with intravenous thrombolysis who present within 4.5 hours of symptoms, literature has reported excellent improvement of symptoms without complications.[2][5][14] The theory is that spinal cord strokes can be treated similarly to cerebral strokes, but further studies are needed as it is not considered the standard of care.[5]
Treatment is usually supportive and focused on treating acute and late symptoms or complications.[5]
- Fluid/vasopressors: for neurogenic hypotension.
- Mechanical ventilation: for the involvement of phrenic nerve and dysfunction of the diaphragm in high cervical lesions
- Bladder catheterization: for neurogenic urinary retention.
- Deep venous thrombosis prevention prophylaxis.
- Pressure sores prevention.
- Gastrointestinal stress ulcer prevention.
- Physical, occupational, and psychological therapy
The goal of the treatment of ASAS focuses on treating the underlying etiology behind the development of ASAS. Current treatment follows the same guidelines for cerebral ischemia, atherosclerotic vascular disease, and acute traumatic spinal cord injury.[3] If the underlying cause is treatable, the promptness of treatment is the most important factor for prognosis.[1] The most common cause of ASAS is aortic surgery; hence, treatment should point towards preventing ASAS. There is an established protocol to prevent ASAS in aortic surgery. It consists of increasing blood flow to the anterior spinal artery by increasing mean arterial pressure and placing a lumbar drain to remove CSF and decrease pressure at the spinal cord.[3][5] It is essential to rule out AVM or aortic dissection as the underlying cause before thrombolysis due to contraindication in these cases.[2]
Differential Diagnosis
- Transverse myelitis: slower onset
- Multiple sclerosis: similar MRI findings but the patient may present cranial and ocular manifestations
- Venous congestive myelopathy: prominent enlarged pial veins with central and peripheral white matter changes
- Spinal cord neoplasms: slower onset
- Spinal cord arteriovenous malformations
- Disk herniation
- Epidural hematoma
- Epidural abscess
- Spinal cord compressive pathologies
- Central cord syndrome
- Dorsal cord syndrome
- Brown-Séquard syndrome
- Guillain-Barré syndrome
- Conus medullaris syndrome
- Cauda equina syndrome
Prognosis
The long-term prognosis of ASAS is only briefly described in the literature. It is poor in general, but one study mentioned that spinal strokes have a better prognosis than cerebral strokes, which also involve cognitive damage.[2][3] The primary etiology behind ASAS is important for prognosis. Less common etiologies, such as disc herniation if treated rapidly and adequately, can result is good to full complete recovery of symptoms.[1][5] Spinal cord infarction has an overall mortality rate of 9 to 23% and usually occurs shortly after the injury.[5] A review of patients with spinal cord infarction reported that 24% did not improve, and only 20% improved significantly.[27] Two other studies reported that only 1-5% recovered completely.[28][29]
Most survivors will have varying degrees of functional motor and sensory dysfunction.[5] Patients with more severe presentation and without improvement in the first 24 hours after insult, resulting in a poorer prognosis.[3] In less severe cases, favorable outcomes with improvement in neurological deficits are possible with a small portion of patients regaining the ability to walk. In others, functional improvement can develop slowly over the years.[5]
Multiple factors have correlations with better or worse prognoses.[30][31][32]
- Etiology: aortic dissection/rupture and high cervical lesions have a greater risk of death.
- Symptom severity at initial presentation: Those with more severe initial presenting symptoms have the worst outcomes.
- Improvement in the first 24 hours: Those with no improvement during the initial 24 hours have the worst outcomes.
- Female sex correlates with poorer outcomes.
- Old age is associated with poorer outcomes.
Complications
Patients with ASAS develop multiple complications associated with the condition. Complications arise due to the highly incapacitating injury to the spinal cord. The complications and presenting symptoms are dependant on the level in the spinal cord that the insults occur. The majority of life-threatening complications in ASAS show shortly after the ischemic insult or during the hospitalization period:[5][6]
- Hypotension: involvement of lateral horns (T1-L2) may lead to shock with fatal consequences if not treated/corrected.
- Respiratory failure: high cervical-level injury will involve the phrenic nerve (C3-C5), which may lead to prolonged mechanical ventilation.
- Bradycardia: this may lead to cardiac arrest for which patients may require atropine or even permanent electrical pacing.
- Infections (urinary tract infections, pneumonia, bacteremia)
- Electrolyte imbalances
- Renal failure
- Depression
ASAS prognosis for recovery is dismal with poor outcomes. Many complications develop as most patients do not recover their motor and sensory dysfunction and have prolonged immobilization.[2][3][5][32][33][34]
- Permanent para or quadra paresis or plegia
- Deep venous thrombosis
- Pulmonary embolism
- Pressure ulcers with a 50% lifetime risk
- Neurogenic bowel/bladder
- Sexual dysfunction
- Neuropathic pain
- Gastrointestinal stress ulcers
- Gastrointestinal dysmotility
- Chronic pain in 79% of the patients
- Increased risk of osteoporosis due to prolonged immobilization
- Spasticity
- Psychological depression and other conditions
- Economic issues
- Dependency on others in activities of daily living
Postoperative and Rehabilitation Care
The successful long-term management of ASAS is dependant on adequate and comprehensive rehabilitation before and after discharge.[6] Rehabilitation focuses on improving the quality of life, independence, and prevention of secondary complications.[3] Although important, the rehabilitation is not only focused on improving motor function but also consists of physical, occupational, vocational, and neuropsychological therapies.[3][6]
Consultations
- Neurology
- Neuro-radiologist
- Neuro-intensivist
- Pneumologist
- Urologist
- Psychologist
- Psychiatrist
- Social work
- Physical medicine and rehabilitation
Deterrence and Patient Education
Although ASAS is unpreventable and usually non-reversible, the most common underlying causes that lead to the development of ASAS are preventable such as aortic aneurysm/dissection and atherosclerotic disease. The patient's education should focus on primary disease prevention, especially at preventing major risk factors such as hypertension, dyslipidemia, diabetes mellitus, and smoking to promote a healthier lifestyle, including healthy nutrition, exercise, and smoking cessation. If a patient develops morbidities that are risk factors, it is crucial to start medical management to control those risk factors. The strict management of risk factors helps to prevent recurrence or worsening functionality.[5]
Pearls and Other Issues
- ASAS is caused by ischemia at the anterior two-thirds of the spinal cord due to anterior spinal artery reduced blood flow.
- The most common etiology is aortic surgery.
- For preventable conditions such as aortic aneurysm or dissection, patient education about preventable risk factors is essential.
- Presents as acute back pain at the level of injury
- The patient shows bilateral motor dysfunction, loss of pain and temperature, with preservation of proprioception and vibratory sense.
- May have autonomic dysfunction.
- MRI is the gold standard for diagnosis.
- No acute treatment is available; management focuses on treating presenting symptoms and complications.
- A more severe presentation without acute improvement results in the worst prognosis
- Most patients remain functionally incapacitated.
- Physical and emotional support is essential.
Enhancing Healthcare Team Outcomes
Although rare, ASAS is a severe, incapacitating syndrome that usually affects multiple organs and produces poor outcomes. A team approach to managing this condition after the arrival in the emergency room results in the best possible outcome. The emergency room physician must do a rapid recognition of the problem and obtain imaging that confirms the diagnosis, which leads to early treatment.
The interprofessional team is necessary for the management of ASAS. Nurses are vital members whose role is crucial in identifying and preventing complications (pressure ulcers, urinary retention). Pharmacists, whose role is no less important since patients with ASAS, especially during the acute period, generally need multiple medications, resulting in polypharmacy. Physical and occupational therapists whose role is an essential factor in determining a patient's prognosis, especially for the long-term. Additionally, social workers and the different physicians treat the patient for mental, social, economic, and physical disabilities. In patients with spinal cord infarction, functional independence and overall lifespan have improved due to improvements in all healthcare fields.[5]