Introduction
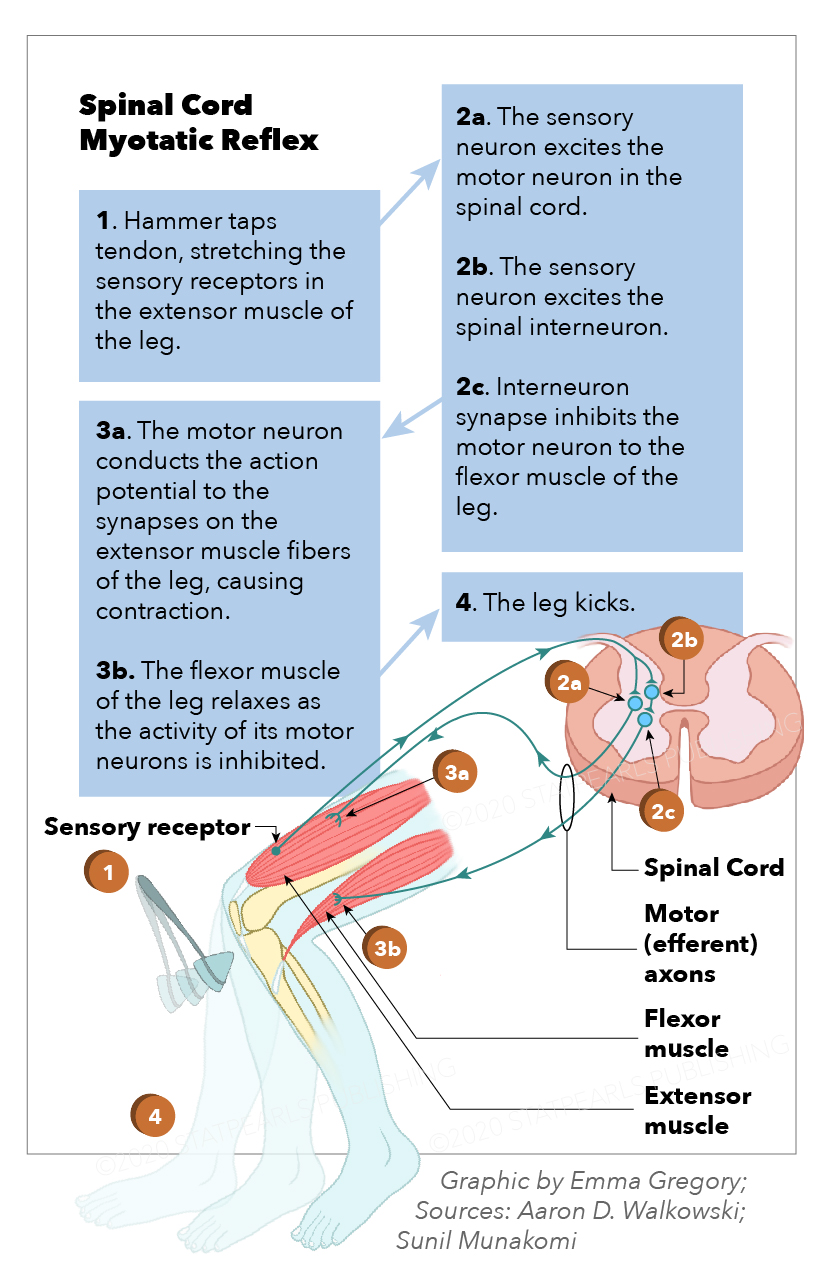
The myotatic reflex is monosynaptic, induced by the sudden passive muscle belly fiber stretch. It produces a muscle contraction in the agonist muscle or muscle group with simultaneous antagonist muscle (or muscle group) relaxation.[1] As with all reflexes, it is involuntary, reproducible, and likely positively selected evolutionarily, specifically to prevent the forceful stretching or pulling of a muscle beyond its normal passive range. This reflex also has utility in helping maintain an upright posture by detecting postural muscle group stretch and reflexively correcting accordingly in the common cases of leaning or postural instability, which conserves expended energy.[2] Additionally called the deep tendon, stretch, and jerk reflex, the myotatic reflex has been a fundamental and essential part of neurological examination since the work by Joseph Babinski, Wilheim Erb, and William Gowers in the late 1800s.[3][4] See Image. Spinal Cord Myotatic Reflex.
Structure and Function
The neuroanatomy of the myotatic reflex follows a model of 5 main paired neurological structures and actions. These structures are the muscle spindle, primary afferent sensory neuron, inhibitory interneuron, efferent alpha motor neuron, and gamma motor neuron. Before the reflex initiates, it must be induced either by the unconscious passive stretch of postural muscles or performed clinically by a medical provider. Tools such as reflex hammers produce a sudden passive stretch to a tendon or muscle belly.
1: The Muscle Spindle. The myotatic reflex begins when mechanical stretch gets detected by a fusiform-shaped microstructure called the muscle spindle, located within the muscle belly and lying between and parallel with the extrafusal striated muscle fibers of a muscle or muscle group. Muscle spindles consist of an outer connective tissue sheath containing spiral treads, 6 to 8 intrafusal specialized muscle fibers, and nerve endings.[5] Because the positioning of the intrafusal fibers of the muscle spindle is parallel with the extrafusal muscle fibers, they stretch and contract with the extrafusal striated muscle fibers. Muscle spindles monitor the elongation velocity of surrounding muscle fibers. When the muscle is lengthened too far or too quickly, the myotatic reflex initiates.[6] Though every skeletal muscle contains several muscle spindles, muscles that provide fine movement contain a larger concentration of muscle spindles than muscles more utilized for coarse movement and posture.[7]
Intrafusal muscle fibers are organized into 3 main types based on shape, function, and innervation, with the majority of spindle fibers containing all 3 types of intrafusal fibers.
Nuclear Chain Fibers: Muscle spindles typically have 2 to 7 nuclear chain fibers that detect and signal information about the static length of the chain fiber itself and thus the surrounding extrafusal muscle fibers. These intrafusal fibers are thinner and shorter than other types of intrafusal fibers and contain fewer linearly-oriented nuclei, giving them the name nuclear chain fibers. They receive innervation by all 3 types of intrafusal nerves: Ia, II, and gamma motor neurons (GMN). This topic covers these nerves and their functions in a later section.[5]
Static Nuclear Bag Fibers: Named due to their centrally clustered nuclei, these fibers also relay information on the static length of the intrafusal and extrafusal muscle fibers. Longer than the chain fibers, bag fibers extend beyond the muscle spindle sheath and attach longitudinally to the connective tissue of the adjacent extrafusal muscle fibers. Ia, II, and GMNs innervate these fibers.[5]
Dynamic Nuclear Bag Fibers: Though structurally similar to static nuclear bag fibers, these serve a different functional purpose of relaying information on the velocity (rate of change in length) of muscle stretch. Ia and GMNs only innervate these fibers.[5]
The innervation of muscle spindle intrafusal fibers grossly grouped into 2 main categories: afferent nerves (type 1a and II) that relay information about the intrafusal fibers to the spinal cord, and efferent nerves (GMNs) that project from the spinal cord to the intrafusal fibers and function to change the length of the intrafusal fibers for maintained reflex sensitivity.
Group 1a Afferents: Also called primary afferents, this nerve type has specialized annulospiral nerve endings that wrap around the central nuclei-containing portion of all 3 types of intrafusal fibers and relay information on both the static length and velocity of these intrafusal fibers. Because the action potential relayed to the spinal cord from group Ia afferent is more rapid than group II afferents and is both length and velocity-dependent, the action potential sent along Ia fibers is the primary afferent signal utilized in the myotatic reflex.[6]
Group II Afferents: Also called secondary afferents, these have specialized flower spray endings that attach to the ends of only nuclear chain fibers and static nuclear bag fibers and relay a much slower, less dynamic action potential dependent on intrafusal muscle length only, thus not utilized in the myotatic reflex.[6]
Gamma Motor Neurons: An in-depth explanation of the GMN appears later as it functions later in the myotatic reflex arc.
2: The Afferent Sensory Neuron. An afferent action potential is then carried along a 1a primary sensory neuron, passing its cell body located paravertebrally at the dorsal root ganglion and entering the spinal cord via the dorsal root(s) at the corresponding spinal levels (for example, L2-L4 for the patellar reflex). After entering the spinal cord posteriorly through the dorsal horn gray matter, the afferent primary sensory neuron’s axon bifurcates before synapsing.[1]
3: The Interneuron. Once separated inside the spinal cord, 1 afferent sensory neuron axon travels anteriorly through the spinal cord to the ipsilateral ventral horn to synapse directly with an activating alpha motor neuron (AMN) using the neurotransmitter glutamate within the lamina IX spinal cord location. The other synapses with an inhibitory interneuron using the neurotransmitter acetylcholine in lamina III of the dorsal horn. This inhibitory interneuron exits the gray matter laterally, ascend or descend the spinal cord via the white matter of the propriospinal tract, also called the fasciculus propius, and re-enter the gray matter to synapse with and inhibit a separate AMN in the lamina IX of the anterior horn using the neurotransmitter glycine at the corresponding spinal level of the antagonist muscle group. For example, an action potential from the quadriceps enters the spinal cord and synapse with an interneuron at the L4 level, then descend along the interneuron to inhibit the activation AMNs to the level of the hamstrings’ innervation at L5 or S1.[8][9]
4: The Efferent Alpha Motor Neuron. After synapsing with the 1a afferent sensory fibers, an action potential from the activated AMN exits the spinal cord via the ventral root(s) at the same spinal level. It travels along an efferent AMN axon that innervates the extrafusal fibers via attachment to the motor endplate of the same muscle or muscle group from which the reflex is initiated. With the antagonist muscle AMN action potential inhibited by the interneuron, no reflexive action potential travels along the AMN of the antagonist muscle or muscle group, which eventually induces a brisk, reflexive contraction in the agonist muscle or muscle group while allowing relaxation in the antagonist.
5: The Gamma Motor Neuron. These efferent nerves also project from the lamina IX portion of the anterior horn of the spinal cord to the polar regions of the intrafusal fibers. They are responsible for the contraction of the intrafusal fibers, functioning to improve the sensitivity of the intrafusal fibers and, consequently, the myotatic reflex. These are called fusomotor neurons. Though GMNs are much smaller in diameter than AMNs, their function is highly important as after the intrafusal fibers have stretched and the Ia afferents have sent the action potential to the spinal cord, signaling the need for a reflexive contraction; the intrafusal fibers also need to contract, to maintain their sensitivity to their extrafusal environment. This simultaneous firing of the AMN and GMN is called alpha-gamma coactivation.[10]
Nerves of Reflexes Commonly Encountered Clinically
The specific nerves utilized in the myotatic reflex depend on where the examiner initiates the reflex. Additionally, there can be slight anatomical differences between individuals in the innervation of their muscle groups. Below are the most commonly elicited myotatic reflex arcs and the most frequently occurring neuroanatomy associated with each circuit.
The biceps brachii reflex is elicited by inducing rapid stretch in the biceps brachii tendon, sending an afferent action potential to the spinal cord via the musculocutaneous nerve and dorsal nerve roots C5-C6 (primarily C5). A post-synaptic efferent action potential returns via ventral nerve roots C5-C6 to the same muscle group, provoking a reflexive muscle contraction of the biceps. An action potential is inhibited in the ventral nerve roots C6-C8, allowing for relaxation of the antagonist muscle group, the triceps.
The triceps reflex is elicited by inducing rapid stretch in the triceps tendon, sending an afferent action potential to the spinal cord via the radial nerve and dorsal nerve roots C6-C8 (primarily C7). A post-synaptic efferent action potential returns via ventral nerve roots C6-C8 to the same muscle group, provoking a reflexive muscle contraction of the triceps. An action potential is inhibited in the ventral nerve roots C5-C6, allowing for relaxation of the antagonist muscle group, the biceps.
The brachioradialis reflex is elicited by inducing rapid stretch in the brachioradialis tendon, sending an afferent action potential to the spinal cord via the radial nerve and dorsal nerve roots C5-C6 (primarily C6). A post-synaptic efferent action potential returns via ventral nerve roots C5-C6 to the same muscle group, provoking a reflexive muscle contraction of the brachioradialis. An action potential is inhibited in the ventral nerve roots C6-C8, allowing for relaxation of the antagonist muscle group, the triceps.
The extensor digitorum reflex also called the Braunecker-Effenberg reflex, is elicited by inducing rapid stretch in the common extensor digitorum tendon of the forearm, sending an afferent action potential to the spinal cord via the radial/posterior interosseous nerves and dorsal nerve roots C6-C7. A post-synaptic efferent action potential returns via ventral nerve roots C6-C7 to the same muscle group, provoking a reflexive muscle contraction of the extensor digitorum. An action potential is inhibited in the ventral nerve roots C8-T1, allowing for relaxation of the antagonist muscle group, the flexor digitorum profundus.
The quadriceps femoris reflex, also called the patellar reflex, is elicited by inducing rapid stretch in the common quadriceps tendon distal to the patella (technically the patellar ligament, but in this functional context, the quadriceps femoris tendon), sending an afferent action potential to the spinal cord via the femoral nerve and dorsal nerve roots L2-L4, (primarily L4). A post-synaptic efferent action potential returns via ventral nerve roots L2-L4 to the same muscle group, provoking a reflexive muscle contraction of the quadriceps. An action potential is inhibited in the ventral nerve roots L5-S1, allowing for relaxation of the antagonist muscle group, the hamstring muscle group.
The Achilles reflex also called the ankle jerk reflex, is elicited by inducing rapid stretch in the calcaneal tendon, found distal to the triceps surae, sending an afferent action potential to the spinal cord via the femoral nerve and dorsal nerve roots S1-S2 (primarily S1). A post-synaptic efferent action potential returns via ventral nerve roots S1-S2 to the same muscle group, provoking a reflexive muscle contraction of the triceps surae. An action potential is inhibited in the ventral nerve roots L2-L4, allowing for relaxation of the antagonist muscle group, the tibialis anterior muscle group.[11]
Embryology
The most basic type of motor coordination is a spinal reflex. From the neuroectoderm, the neural plate (central thickening of cells) originates, which subsequently turns into a neural groove and neural tube. During the formation of the tube, the cells that form the neural crests differentiate, bordering on the ectoderm (at the level of the neural folds), and from the neural tube originates the spinal cord and the encephalon. In contrast, from the neural crests originate the peripheral nervous system and the spinal ganglia.
Between the fifth and seventh week of gestation, the myotatic reflex of primitive stretching begins to develop.
Clinical Significance
In the clinical setting, the myotatic reflex receives a grade on a scale, with a number score given based on the physical quality of the muscle contraction and subsequent limb flexion or extension elicited by the initial induced mechanical stretch. This score, ranging from 0 to 4, can help clinicians diagnose neurological and system diseases, accurately predict disease outcomes, and grossly locate neurological lesions.
Myotatic Reflex Scoring:
- 0: No contraction. This lack of response is always considered pathological, though not definitive, for any specific neurological disease.
- 1: Weak, slight response. This response can be pathological or physiological.
- 2: Brisk reflex. The normal, non-pathological reflex.
- 3: Increased, greater than brisk response, with the absence of clonus. This response can be pathological or physiological.
- 4: Elicitation of clonus. Characterized by repeated rhythmic contractions in the agonist muscle group, clonus is always considered a pathological finding.
In addition to this basic reflex scoring system, the number score designated to an elicited reflex can also have a “+“ or “-” to additionally help characterize reflexes that the provider considers slightly greater than or less than a particular numerical value.
The myotatic reflex has vital utility in helping physicians localize a neurological lesion or pathology to either the central nervous system (CNS) or the peripheral nervous system (PNS). Hyperreflexia, designated by a reflex score of 3 or 4, is often a supportive exam finding in patients with upper motor neuron (UMN) lesions or injury, grossly meaning an injury to the CNS. A UMN lesion is also frequently concomitant with exam findings of muscle spasticity and the absence of muscle fasciculations and is commonly associated with conditions such as paraplegia, tetraplegia, hemiplegia, and diplegia.[12][13][14] Hyporeflexia, designated by a reflex score of 0 or 1, is often a supportive exam finding in patients with lower motor neuron (LMN) lesions or injury, grossly meaning damage to the PNS, for example, the anterior horn cells of the spinal cord, nerve roots, peripheral nerves, or the neuromuscular junction or the muscle itself. An LMN lesion is also frequently concomitant with exam findings of muscle flaccidity, hypotonia, and fasciculations and is commonly associated with conditions such as myopathies and neuropathies.
Hypo- and hyperreflexia can also be normal physiological exam variations as a result of aging, with children and young individuals commonly having more brisk reflexes and elderly individuals having non-pathological decreased reflexes. These same findings can also be representative of metabolic dysfunction, including electrolyte imbalance, thyroid dysfunction, liver and kidney disease, medication toxicity, and neoplastic processes.