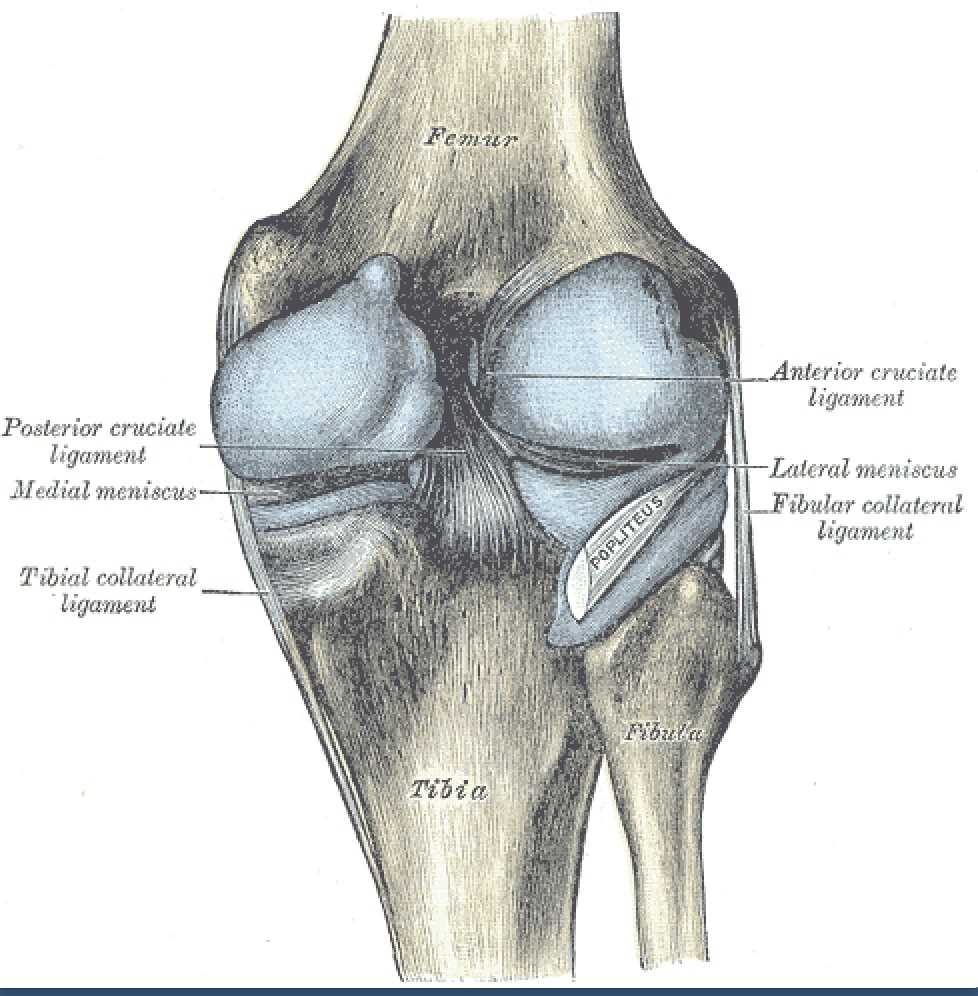
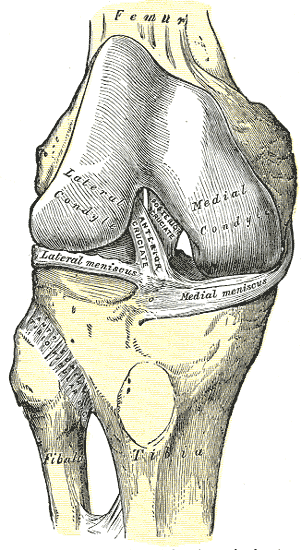
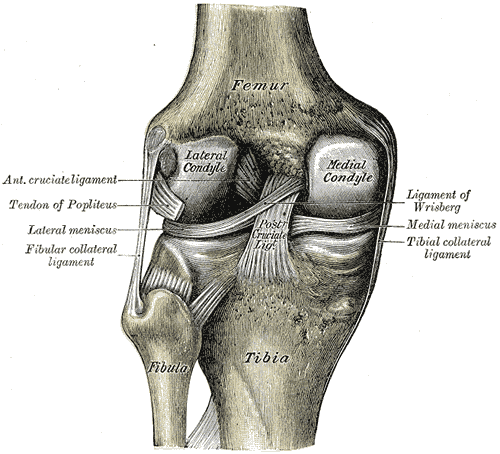
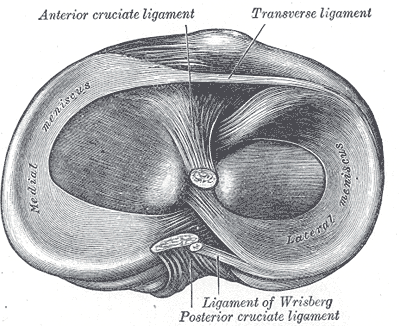
[1]
Duthon VB, Barea C, Abrassart S, Fasel JH, Fritschy D, Ménétrey J. Anatomy of the anterior cruciate ligament. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2006 Mar:14(3):204-13
[PubMed PMID: 16235056]
[2]
Markatos K, Kaseta MK, Lallos SN, Korres DS, Efstathopoulos N. The anatomy of the ACL and its importance in ACL reconstruction. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2013 Oct:23(7):747-52. doi: 10.1007/s00590-012-1079-8. Epub 2012 Sep 22
[PubMed PMID: 23412211]
[3]
Arnoczky SP. Anatomy of the anterior cruciate ligament. Clinical orthopaedics and related research. 1983 Jan-Feb:(172):19-25
[PubMed PMID: 6821989]
[4]
Girgis FG, Marshall JL, Monajem A. The cruciate ligaments of the knee joint. Anatomical, functional and experimental analysis. Clinical orthopaedics and related research. 1975 Jan-Feb:(106):216-31
[PubMed PMID: 1126079]
[5]
Ellison AE,Berg EE, Embryology, anatomy, and function of the anterior cruciate ligament. The Orthopedic clinics of North America. 1985 Jan;
[PubMed PMID: 3969275]
[6]
Smith BA, Livesay GA, Woo SL. Biology and biomechanics of the anterior cruciate ligament. Clinics in sports medicine. 1993 Oct:12(4):637-70
[PubMed PMID: 8261518]
[7]
Dienst M, Burks RT, Greis PE. Anatomy and biomechanics of the anterior cruciate ligament. The Orthopedic clinics of North America. 2002 Oct:33(4):605-20, v
[PubMed PMID: 12528904]
[8]
Yahagi Y, Horaguchi T, Iriuchishima T, Suruga M, Iwama G, Aizawa S. Correlation between the mid-substance cross-sectional anterior cruciate ligament size and the knee osseous morphology. European journal of orthopaedic surgery & traumatology : orthopedie traumatologie. 2020 Feb:30(2):291-296. doi: 10.1007/s00590-019-02552-x. Epub 2019 Sep 24
[PubMed PMID: 31552484]
Level 2 (mid-level) evidence
[9]
Markolf KL, Mensch JS, Amstutz HC. Stiffness and laxity of the knee--the contributions of the supporting structures. A quantitative in vitro study. The Journal of bone and joint surgery. American volume. 1976 Jul:58(5):583-94
[PubMed PMID: 946969]
[10]
Beynnon BD, Fleming BC, Labovitch R, Parsons B. Chronic anterior cruciate ligament deficiency is associated with increased anterior translation of the tibia during the transition from non-weightbearing to weightbearing. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2002 Mar:20(2):332-7
[PubMed PMID: 11918313]
[11]
Zantop T, Herbort M, Raschke MJ, Fu FH, Petersen W. The role of the anteromedial and posterolateral bundles of the anterior cruciate ligament in anterior tibial translation and internal rotation. The American journal of sports medicine. 2007 Feb:35(2):223-7
[PubMed PMID: 17158275]
[12]
Domnick C, Raschke MJ, Herbort M. Biomechanics of the anterior cruciate ligament: Physiology, rupture and reconstruction techniques. World journal of orthopedics. 2016 Feb 18:7(2):82-93. doi: 10.5312/wjo.v7.i2.82. Epub 2016 Feb 18
[PubMed PMID: 26925379]
[13]
Herbort M, Lenschow S, Fu FH, Petersen W, Zantop T. ACL mismatch reconstructions: influence of different tunnel placement strategies in single-bundle ACL reconstructions on the knee kinematics. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2010 Nov:18(11):1551-8. doi: 10.1007/s00167-010-1163-8. Epub 2010 May 12
[PubMed PMID: 20461359]
[14]
Gabriel MT, Wong EK, Woo SL, Yagi M, Debski RE. Distribution of in situ forces in the anterior cruciate ligament in response to rotatory loads. Journal of orthopaedic research : official publication of the Orthopaedic Research Society. 2004 Jan:22(1):85-9
[PubMed PMID: 14656664]
[15]
Fleming BC, Renstrom PA, Beynnon BD, Engstrom B, Peura GD, Badger GJ, Johnson RJ. The effect of weightbearing and external loading on anterior cruciate ligament strain. Journal of biomechanics. 2001 Feb:34(2):163-70
[PubMed PMID: 11165279]
[16]
Beynnon BD, Johnson RJ, Fleming BC, Peura GD, Renstrom PA, Nichols CE, Pope MH. The effect of functional knee bracing on the anterior cruciate ligament in the weightbearing and nonweightbearing knee. The American journal of sports medicine. 1997 May-Jun:25(3):353-9
[PubMed PMID: 9167816]
[17]
Gardner E, O'Rahilly R. The early development of the knee joint in staged human embryos. Journal of anatomy. 1968 Jan:102(Pt 2):289-99
[PubMed PMID: 5643844]
[18]
de Carvalho RT, Ramos LA, Novaretti JV, Ribeiro LM, Szeles PR, Ingham SJ, Abdalla RJ. Relationship Between the Middle Genicular Artery and the Posterior Structures of the Knee: A Cadaveric Study. Orthopaedic journal of sports medicine. 2016 Dec:4(12):2325967116673579. doi: 10.1177/2325967116673579. Epub 2016 Dec 9
[PubMed PMID: 28050573]
[19]
Salaria H, Atkinson R. Anatomic study of the middle genicular artery. Journal of orthopaedic surgery (Hong Kong). 2008 Apr:16(1):47-9
[PubMed PMID: 18453659]
[20]
Scapinelli R. Vascular anatomy of the human cruciate ligaments and surrounding structures. Clinical anatomy (New York, N.Y.). 1997:10(3):151-62
[PubMed PMID: 9135883]
[21]
Arnoczky SP, Rubin RM, Marshall JL. Microvasculature of the cruciate ligaments and its response to injury. An experimental study in dogs. The Journal of bone and joint surgery. American volume. 1979 Dec:61(8):1221-9
[PubMed PMID: 511882]
[22]
Kennedy JC, Alexander IJ, Hayes KC. Nerve supply of the human knee and its functional importance. The American journal of sports medicine. 1982 Nov-Dec:10(6):329-35
[PubMed PMID: 6897495]
[23]
Hogervorst T, Brand RA. Mechanoreceptors in joint function. The Journal of bone and joint surgery. American volume. 1998 Sep:80(9):1365-78
[PubMed PMID: 9759824]
[24]
Marieswaran M, Jain I, Garg B, Sharma V, Kalyanasundaram D. A Review on Biomechanics of Anterior Cruciate Ligament and Materials for Reconstruction. Applied bionics and biomechanics. 2018:2018():4657824. doi: 10.1155/2018/4657824. Epub 2018 May 13
[PubMed PMID: 29861784]
[25]
Anderson MJ, Browning WM 3rd, Urband CE, Kluczynski MA, Bisson LJ. A Systematic Summary of Systematic Reviews on the Topic of the Anterior Cruciate Ligament. Orthopaedic journal of sports medicine. 2016 Mar:4(3):2325967116634074. doi: 10.1177/2325967116634074. Epub 2016 Mar 15
[PubMed PMID: 27047983]
Level 1 (high-level) evidence
[26]
Hewett TE, Myer GD, Ford KR. Anterior cruciate ligament injuries in female athletes: Part 1, mechanisms and risk factors. The American journal of sports medicine. 2006 Feb:34(2):299-311
[PubMed PMID: 16423913]
[27]
Renstrom P, Ljungqvist A, Arendt E, Beynnon B, Fukubayashi T, Garrett W, Georgoulis T, Hewett TE, Johnson R, Krosshaug T, Mandelbaum B, Micheli L, Myklebust G, Roos E, Roos H, Schamasch P, Shultz S, Werner S, Wojtys E, Engebretsen L. Non-contact ACL injuries in female athletes: an International Olympic Committee current concepts statement. British journal of sports medicine. 2008 Jun:42(6):394-412. doi: 10.1136/bjsm.2008.048934. Epub
[PubMed PMID: 18539658]
[28]
Geng B, Wang J, Ma JL, Zhang B, Jiang J, Tan XY, Xia YY. Narrow Intercondylar Notch and Anterior Cruciate Ligament Injury in Female Nonathletes with Knee Osteoarthritis Aged 41-65 Years in Plateau Region. Chinese medical journal. 2016 Nov 5:129(21):2540-2545. doi: 10.4103/0366-6999.192771. Epub
[PubMed PMID: 27779159]
[29]
Price MJ, Tuca M, Cordasco FA, Green DW. Nonmodifiable risk factors for anterior cruciate ligament injury. Current opinion in pediatrics. 2017 Feb:29(1):55-64. doi: 10.1097/MOP.0000000000000444. Epub
[PubMed PMID: 27861256]
Level 3 (low-level) evidence
[30]
Barber-Westin SD, Noyes FR, Galloway M. Jump-land characteristics and muscle strength development in young athletes: a gender comparison of 1140 athletes 9 to 17 years of age. The American journal of sports medicine. 2006 Mar:34(3):375-84
[PubMed PMID: 16282578]
[31]
Alentorn-Geli E, Myer GD, Silvers HJ, Samitier G, Romero D, Lázaro-Haro C, Cugat R. Prevention of non-contact anterior cruciate ligament injuries in soccer players. Part 2: a review of prevention programs aimed to modify risk factors and to reduce injury rates. Knee surgery, sports traumatology, arthroscopy : official journal of the ESSKA. 2009 Aug:17(8):859-79. doi: 10.1007/s00167-009-0823-z. Epub 2009 Jun 9
[PubMed PMID: 19506834]