Introduction
Cytogenetic testing is the examination of chromosomes to determine chromosome abnormalities such as aneuploidy and structural abnormalities. A normal human cell contains 23 pairs of chromosomes, including 22 pairs of autosomes and a pair of sex chromosomes (XX or XY). Aneuploidy involves having one or more extra chromosomes (e.g., 47 XX +21, 48 XXXY) or having missing chromosomes (e.g., 45 XO). The most common aneuploidies are Down syndrome (trisomy 21), Edward's syndrome (trisomy 18), Turner syndrome (monosomy X), etc.
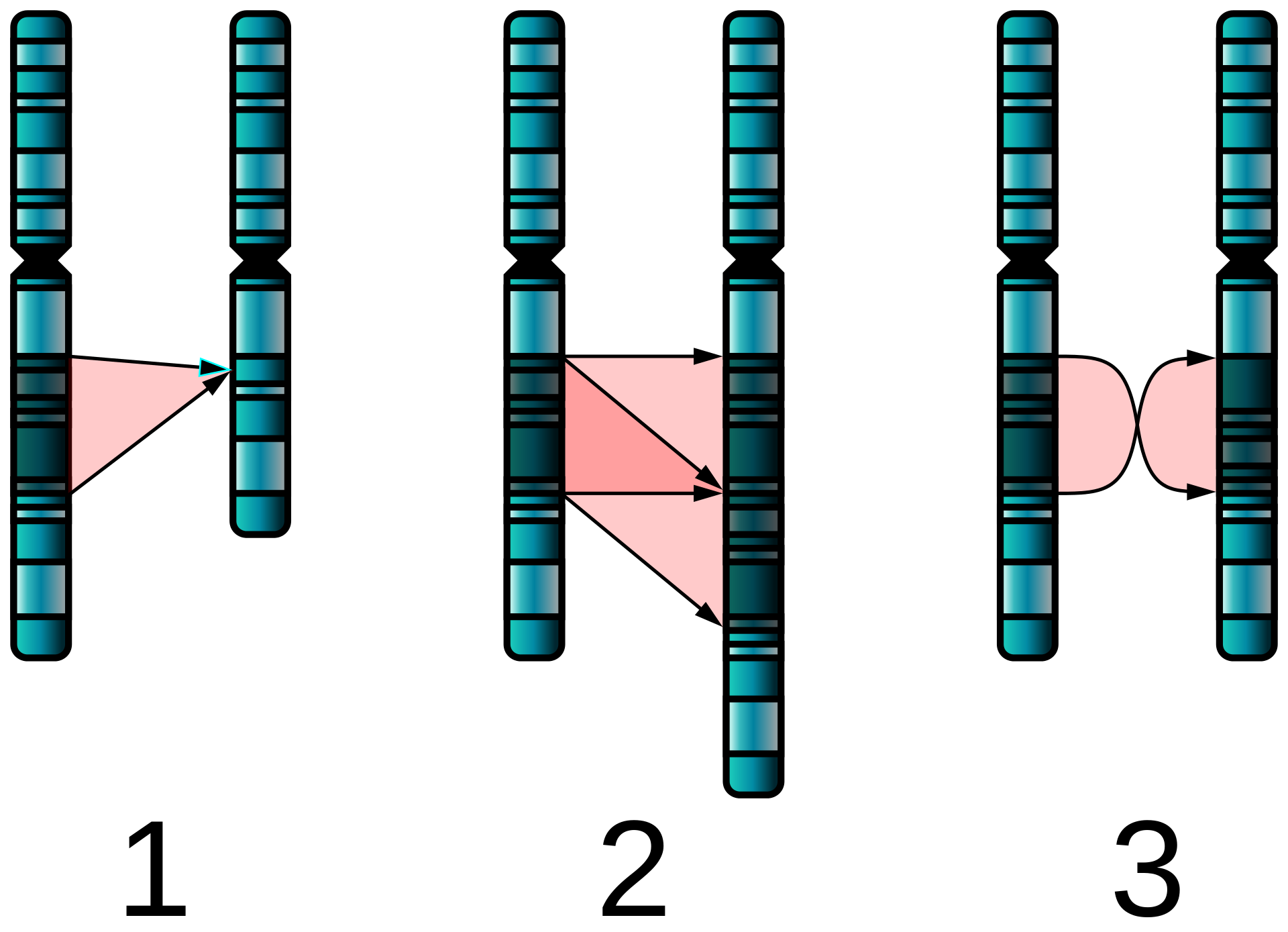
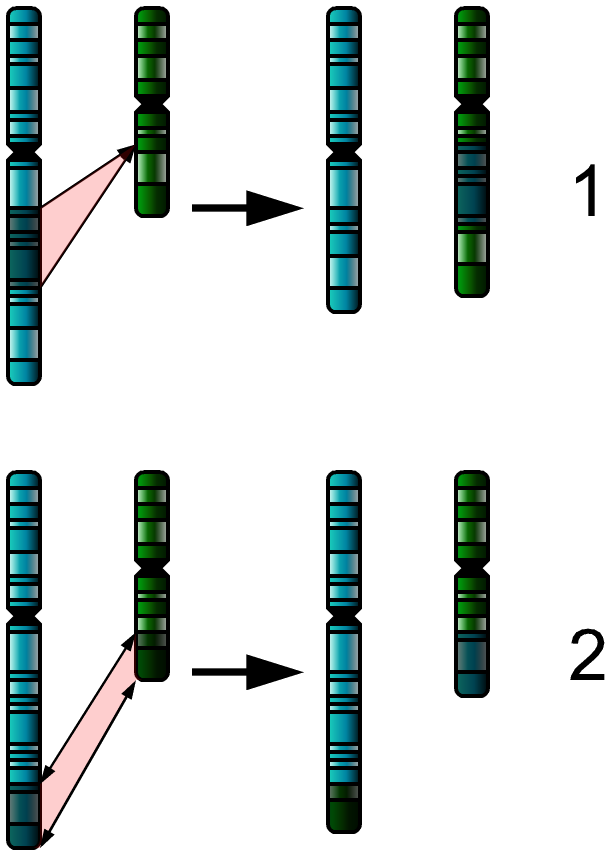
The types of structural abnormalities are [1]:
- Duplication: Part of a chromosome is repeated
- Deletion: Part of a chromosome is missing
- Translocation: Material between two different chromosomes is exchanged (this exchange may be balanced or unbalanced)
- Inversion: Part of the chromosome is inverted within the chromosome
- Insertion: Addition of material from another chromosome.
Cytogenetic testing can be performed in a variety of situations, including solid organ malignancies, hematologic malignancies, congenital diseases. It can be performed prenatally after biochemical screening or ultrasound with abnormal findings. It is also used for parents with multiple miscarriages or significant findings in their pedigree analysis. Postnatally, cytogenetic testing plays a role in distinguishing patients with mosaicism, intellectual disability, autism, or developmental delays.
Cytogenetic analysis can also be utilized to diagnose malignancies, determine appropriate therapy for prognostic stratification. This review explains the types of chromosome analysis, such as karyotyping, fluorescence in situ hybridization (FISH), and chromosomal microarray analysis (CMA).
Specimen Collection
Specimen collection modalities include fine-needle aspiration cytology, fluid sampling and centrifugation, and tissue sampling. A cytogenetic test can be obtained for fetal diagnostic testing as early as 10 weeks of gestation from chorionic villus sampling (CVS), using trophoblast cells or cultured mesenchymal cells. By analyzing cells cultured from amniotic fluid, false mosaicism detected on chorionic villus sampling can be eliminated. Amniocentesis can be performed from the 15th to the 18th week of pregnancy. This requires the cultured sample to grow for one to two weeks.
Another test that may be performed is an examination of the lymphocytes of the fetus by obtaining a sample from the umbilical vein at around the 19th week of gestation. Fibroblasts are the preferred material to obtain from the skin of the abortus. After birth, evaluation of fibroblasts or peripheral blood lymphocytes can be performed. For chromosomal microarray analysis (CMA), genomic DNA can be obtained from peripheral blood, skin fibroblasts, or amniocentesis.[2]
In acute leukemias, bone marrow samples are routinely obtained for conventional karyotype and FISH testing. A timely collection of a bone marrow sample for cytogenetic testing at diagnosis of acute leukemia is essential for adequate prognostic evaluation and treatment selection. In solid tumors and lymphoproliferative disorders, FISH is usually performed using histopathological samples and also impacts prognosis and treatment selection.
Procedures
Karyotyping is one of the most preferred methods to detect structural and numerical abnormalities. Deletions, duplications, balanced or unbalanced translocations, insertions, and inversions are examples of structural abnormalities. Common inherited abnormalities include trisomies of chromosomes 13, 16, 18, or 21, and monosomy, triploidy, or tetraploidy of the X chromosome. Using the G-banding technique, the analysis of 20 metaphase-state cells is the preferred method for karyotyping. If mosaicism is suspected, 30 to 50 metaphases should be analyzed.[3]
Fluorescence in situ hybridization (FISH) detects chromosome abnormalities targeting specific genetic sequences using probes with a fluorescent dye that attaches to complementary DNA. It can be performed in the interphase stage, but it does not analyze the full set of chromosomes, only the sequences targeted by the utilized probes. Therefore, a comprehensive analysis of the case with an adequate differential diagnosis is mandatory before selecting specific probes. FISH studies can detect inversions, insertions, translocations, microdeletions, and microduplications.[3][4] An example of FISH analysis is the detection of inv(16)(p13q22), involving the fusion gene CBFB-MYH11, in acute myeloid leukemia (AML).[4]
Chromosomal microarray analysis (CMA) is a relatively new method to diagnose patients lacking an apparent syndromic phenotype. Diagnoses of cognitive impairment, developmental delay, or autism in suspected patients are increased by 20% using CMA, especially in suspected cases of mosaicism.[5][2] Microarray-based comparative genomic hybridization (aCGH) and single nucleotide polymorphism (SNP) arrays are subtypes of CMA. Similar to FISH, oligonucleotide probes attached to fluorescent dyes are used to label genomic DNA through hybridization.[2]
Indications
Cytogenetic testing is an important diagnostic tool in fetal and genetic medicine, oncology, and hematology.
The main indications of karyotyping and FISH studies in neoplastic diseases are [4]:
- Diagnosis and classification of leukemias
- Estimation of prognosis
- Evaluation of appropriate treatment regimens
- Evaluation of response to treatment by looking for the elimination of cells carrying the abnormal genotype.
The main indications of cytogenetic studies in congenital genetic diseases are:
- Abnormal ultrasound findings
- Abnormal biochemistry results
- Recurrent miscarriages
- Advanced maternal age, particularly >35 years old
- Family history
- Abnormal non-invasive prenatal test results (NIPTS)
- Multiple irrelevant congenital anomalies
Abnormal biochemical findings in maternal serum may include: decreased alpha-fetoprotein (AFP), which might indicate trisomy 21; decreased unconjugated estriol (UE3), increased human chorionic gonadotrophin (hCG), and abnormal inhibin-A levels are also associated with Down syndrome.[6] Additionally, in trisomy 18, AFP, hCG, and UE3 tend to decrease [7].
While most of the findings in an ultrasound examination are insufficient to confirm a diagnosis, femur-length shortening and nuchal skin fold thickening can raise suspicion of trisomy 21.[8][2] Many studies indicate that the risk of trisomy 21, trisomy 18, and trisomy 13 increases with advanced maternal age.[9][10] Taking biochemical tests, ultrasound screening results, and maternal age into consideration, prenatal cytogenetic testing can be performed.[11] A pedigree that contains an individual with a chromosomal anomaly might also have some other individuals with balanced translocations. In these cases, the parents should be tested as there may be unbalanced rearrangement in the embryo.[12] Most of these pregnancies are aborted during the first trimester. More than two pregnancy losses should alert the physician to the possibility of a balanced translocation in one of the parents.[13] Chromosomal studies would allow the diagnosis of any possible unbalanced translocation. Moreover, trisomy recurs in approximately 1% of the subsequent pregnancy in couples diagnosed with trisomy in a previous pregnancy. The chromosomal analysis may be helpful in these cases.[14]
Non-invasive prenatal testing/screening (NIPTS) is a relatively new method that detects chromosomal anomalies using the circulating cell-free fetal DNA present in the mother's plasma.[15] High cfDNA from sex chromosomes and chromosomes 13, 18, and 21 is associated with the respective syndromes. Moreover, it can serve as a method to reveal microdeletion syndromes by detecting an abnormal number of copies.[7]
Potential Diagnosis
As mentioned above, chromosomal analysis can be used to diagnose variable aneuploidies such as monosomy X, trisomies, triploidy (3n/69), and tetraploidy (4n/92). These tests are preferred to diagnose balanced and unbalanced translocations, recurrent microdeletions such as 22q11.2 deletion (DiGeorge syndrome), 15q11.2q13.1 deletion (Prader-Willi/Angelman), or 5p15.3 microdeletion (cri-du-chat syndrome). In particular, CMA may be used to diagnose the etiology of developmental delay in patients without a known cause. It is also helpful to analyze autism spectrum disorder or intellectual disability.[16]
Translocation between the long arms of chromosomes 9 and 22 t(9;22)(q34;q11) was the first demonstration that cancer could result from a genetic abnormality. This aberration was named the Philadelphia chromosome.[4][17] It is key for the diagnosis of Chronic Myeloid Leukemia (CML), and may also occur in acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). This translocation results in the BCR-ABL fusion protein with constitutive tyrosine kinase activity.[4]
Other significant abnormalities include translocation (15;17) in acute promyelocytic leukemia (APL). This subset of AML responds well to all-trans retinoic acid (tretinoin), highlighting the role of cytogenetic testing in determining the appropriate treatment. Additionally, characteristic translocations in lymphomas include t(8;14) in Burkitt lymphoma, t(14;18) in follicular lymphoma, t(11;14) in mantle cell lymphoma. Translocation (12;21) in pediatric ALL and inversion (16) in AML are examples of aberrations that confer favorable prognosis, whereas inversion(3) and complex karyotype, defined as 3 or more concurrent chromosomal abnormalities, are examples of adverse prognosis in AML.
Normal and Critical Findings
Each human cell holds 22 pairs of autosomes and a pair of sex chromosomes (female: XX, male: XY). In karyotyping, autosomes are sorted by size. Critical findings in conventional karyotyping reveal aneuploidies that involve having one or more extra chromosomes or having missing chromosomes and structural abnormalities such as translocation, deletion, duplication, inversion, insertion. These findings help to diagnose congenital diseases, specific syndromes, and solid organ and hematologic malignancies.
Interfering Factors
An estimation of the pre-test probability of any potential diagnosis is paramount to enable judicious use of cytogenetic testing and avoid a "shotgun-testing" approach, especially considering the expensive nature of these tests. Lack of appropriate differential diagnosis or fixation on a particular diagnosis can lead to inappropriate testing or confirmation bias upon receiving testing results.
For conventional karyotyping, the need for metaphase-stage cells requires cell division in the cultured sample. Lack of adequate preparation or cell types that are not prone to growing in vitro without a specific mean may impede adequate testing. Keeping these pitfalls in mind allows efficient use of cytogenetic analysis and allows a full realization of such testing benefits.
Complications
Risks related to chorionic villi sampling are particularly higher before 10 weeks of gestation. While the percentage of complications in chorionic villi sampling is approximately 1%, the rate is lower (0.5%) in amniocentesis. Chorionic villus sampling allows the patient to contemplate pregnancy termination earlier [2][18]. Amniocentesis is related to the rupture of the amniotic sac, amniotic fluid leakage, and chorioamnionitis.
Patient Safety and Education
Patients must be educated about a small probability of false positives during cytogenetic analyses. They must also be informed about the appropriate timing, precautions, and any risks associated with the sampling process.
Clinical Significance
Cytogenetic testing provides us with an opportunity to improve the management of congenital disorders, blood malignancies, and solid tumors. Parents can be counseled about expectations of a certain congenital disorder, review fetal and maternal risks, and even decide on the continuation of the pregnancy. The risks involved in the termination of a pregnancy are higher without an early cytogenetic diagnosis. Proper assistance for a child with a congenital disorder may include screening for cardiac defects, hearing, and vision assessments, and specialized education, which may not be adequately offered without a timely diagnosis.
Correct diagnosis, prognostic evaluation, and improved therapeutic options often require appropriate cytogenetic testing in acute leukemias and other forms of cancer. Broader availability of diagnostic tools is key for the advancement of cancer management and survivorship.