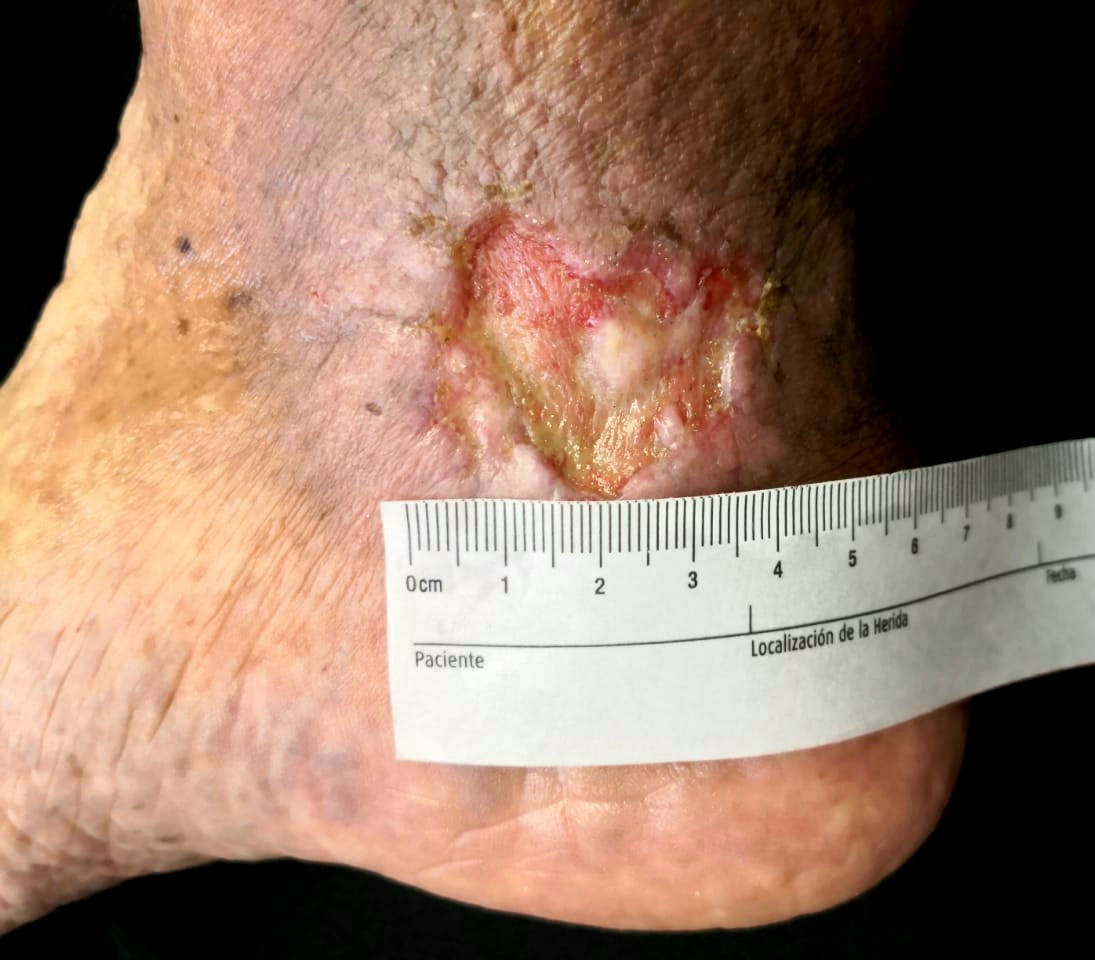
[1]
Bonkemeyer Millan S, Gan R, Townsend PE. Venous Ulcers: Diagnosis and Treatment. American family physician. 2019 Sep 1:100(5):298-305
[PubMed PMID: 31478635]
[2]
Korstanje MJ. Venous stasis ulcers. Diagnostic and surgical considerations. Dermatologic surgery : official publication for American Society for Dermatologic Surgery [et al.]. 1995 Jul:21(7):635-40
[PubMed PMID: 7606378]
[3]
Molnar JA, Vlad LG, Gumus T. Nutrition and Chronic Wounds: Improving Clinical Outcomes. Plastic and reconstructive surgery. 2016 Sep:138(3 Suppl):71S-81S. doi: 10.1097/PRS.0000000000002676. Epub
[PubMed PMID: 27556777]
Level 2 (mid-level) evidence
[4]
Xie T, Ye J, Rerkasem K, Mani R. The venous ulcer continues to be a clinical challenge: an update. Burns & trauma. 2018:6():18. doi: 10.1186/s41038-018-0119-y. Epub 2018 Jun 15
[PubMed PMID: 29942813]
[5]
Santler B, Goerge T. Chronic venous insufficiency - a review of pathophysiology, diagnosis, and treatment. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2017 May:15(5):538-556. doi: 10.1111/ddg.13242. Epub
[PubMed PMID: 28485865]
[6]
Nelson EA, Adderley U. Venous leg ulcers. BMJ clinical evidence. 2016 Jan 15:2016():. pii: 1902. Epub 2016 Jan 15
[PubMed PMID: 26771825]
[7]
Phillips CJ, Humphreys I, Thayer D, Elmessary M, Collins H, Roberts C, Naik G, Harding K. Cost of managing patients with venous leg ulcers. International wound journal. 2020 Aug:17(4):1074-1082. doi: 10.1111/iwj.13366. Epub 2020 May 7
[PubMed PMID: 32383324]
[8]
Guest JF, Fuller GW, Vowden P. Venous leg ulcer management in clinical practice in the UK: costs and outcomes. International wound journal. 2018 Feb:15(1):29-37. doi: 10.1111/iwj.12814. Epub 2017 Dec 15
[PubMed PMID: 29243398]
[9]
Barnsbee L, Cheng Q, Tulleners R, Lee X, Brain D, Pacella R. Measuring costs and quality of life for venous leg ulcers. International wound journal. 2019 Feb:16(1):112-121. doi: 10.1111/iwj.13000. Epub 2018 Oct 5
[PubMed PMID: 30289621]
Level 2 (mid-level) evidence
[10]
Rice JB, Desai U, Cummings AK, Birnbaum HG, Skornicki M, Parsons N. Burden of venous leg ulcers in the United States. Journal of medical economics. 2014 May:17(5):347-56. doi: 10.3111/13696998.2014.903258. Epub 2014 Mar 24
[PubMed PMID: 24625244]
[11]
Olin JW, Beusterien KM, Childs MB, Seavey C, McHugh L, Griffiths RI. Medical costs of treating venous stasis ulcers: evidence from a retrospective cohort study. Vascular medicine (London, England). 1999:4(1):1-7
[PubMed PMID: 10355863]
Level 2 (mid-level) evidence
[12]
Davies AH. The Seriousness of Chronic Venous Disease: A Review of Real-World Evidence. Advances in therapy. 2019 Mar:36(Suppl 1):5-12. doi: 10.1007/s12325-019-0881-7. Epub 2019 Feb 13
[PubMed PMID: 30758738]
Level 3 (low-level) evidence
[13]
Darwin E, Liu G, Kirsner RS, Lev-Tov H. Examining risk factors and preventive treatments for first venous leg ulceration: A cohort study. Journal of the American Academy of Dermatology. 2021 Jan:84(1):76-85. doi: 10.1016/j.jaad.2019.12.046. Epub 2019 Dec 27
[PubMed PMID: 31884088]
[14]
Crawford JM, Lal BK, Durán WN, Pappas PJ. Pathophysiology of venous ulceration. Journal of vascular surgery. Venous and lymphatic disorders. 2017 Jul:5(4):596-605. doi: 10.1016/j.jvsv.2017.03.015. Epub
[PubMed PMID: 28624002]
[15]
Brown J. The role of the fibrin cuff in the development of venous leg ulcers. Journal of wound care. 2005 Jul:14(7):324-7
[PubMed PMID: 16048219]
[16]
Mansilha A, Sousa J. Pathophysiological Mechanisms of Chronic Venous Disease and Implications for Venoactive Drug Therapy. International journal of molecular sciences. 2018 Jun 5:19(6):. doi: 10.3390/ijms19061669. Epub 2018 Jun 5
[PubMed PMID: 29874834]
[17]
Meulendijks AM, Franssen WMA, Schoonhoven L, Neumann HAM. A scoping review on Chronic Venous Disease and the development of a Venous Leg Ulcer: The role of obesity and mobility. Journal of tissue viability. 2020 Aug:29(3):190-196. doi: 10.1016/j.jtv.2019.10.002. Epub 2019 Oct 9
[PubMed PMID: 31668667]
Level 2 (mid-level) evidence
[18]
Raffetto JD. Inflammation in chronic venous ulcers. Phlebology. 2013 Mar:28 Suppl 1():61-7. doi: 10.1177/0268355513476844. Epub
[PubMed PMID: 23482537]
[19]
Rahmani J, Haghighian Roudsari A, Bawadi H, Thompson J, Khalooei Fard R, Clark C, Ryan PM, Ajami M, Rahimi Sakak F, Salehisahlabadi A, Abdulazeem HM, Jamali MR, Mirzay Razaz J. Relationship between body mass index, risk of venous thromboembolism and pulmonary embolism: A systematic review and dose-response meta-analysis of cohort studies among four million participants. Thrombosis research. 2020 Aug:192():64-72. doi: 10.1016/j.thromres.2020.05.014. Epub 2020 May 15
[PubMed PMID: 32454303]
Level 1 (high-level) evidence
[20]
Serra R, Buffone G, de Franciscis A, Mastrangelo D, Molinari V, Montemurro R, de Franciscis S. A genetic study of chronic venous insufficiency. Annals of vascular surgery. 2012 Jul:26(5):636-42. doi: 10.1016/j.avsg.2011.11.036. Epub
[PubMed PMID: 22664280]
[21]
Markovic JN, Shortell CK. Genomics of varicose veins and chronic venous insufficiency. Seminars in vascular surgery. 2013 Mar:26(1):2-13. doi: 10.1053/j.semvascsurg.2013.04.003. Epub
[PubMed PMID: 23932556]
[22]
Charles CA, Tomic-Canic M, Vincek V, Nassiri M, Stojadinovic O, Eaglstein WH, Kirsner RS. A gene signature of nonhealing venous ulcers: potential diagnostic markers. Journal of the American Academy of Dermatology. 2008 Nov:59(5):758-71. doi: 10.1016/j.jaad.2008.07.018. Epub 2008 Aug 20
[PubMed PMID: 18718692]
[23]
Ramelet AA, Hirt-Burri N, Raffoul W, Scaletta C, Pioletti DP, Offord E, Mansourian R, Applegate LA. Chronic wound healing by fetal cell therapy may be explained by differential gene profiling observed in fetal versus old skin cells. Experimental gerontology. 2009 Mar:44(3):208-18. doi: 10.1016/j.exger.2008.11.004. Epub 2008 Nov 20
[PubMed PMID: 19049860]
[24]
Okamoto O, Fujiwara S. Dermatopontin, a novel player in the biology of the extracellular matrix. Connective tissue research. 2006:47(4):177-89
[PubMed PMID: 16987749]
[25]
Gemmati D, Federici F, Catozzi L, Gianesini S, Tacconi G, Scapoli GL, Zamboni P. DNA-array of gene variants in venous leg ulcers: detection of prognostic indicators. Journal of vascular surgery. 2009 Dec:50(6):1444-51. doi: 10.1016/j.jvs.2009.07.103. Epub
[PubMed PMID: 19958990]
[26]
Jacob MP. Extracellular matrix remodeling and matrix metalloproteinases in the vascular wall during aging and in pathological conditions. Biomedicine & pharmacotherapy = Biomedecine & pharmacotherapie. 2003 Jul-Aug:57(5-6):195-202
[PubMed PMID: 12888254]
[27]
Stone RC, Stojadinovic O, Rosa AM, Ramirez HA, Badiavas E, Blumenberg M, Tomic-Canic M. A bioengineered living cell construct activates an acute wound healing response in venous leg ulcers. Science translational medicine. 2017 Jan 4:9(371):. doi: 10.1126/scitranslmed.aaf8611. Epub
[PubMed PMID: 28053158]
[28]
Lal BK. Venous ulcers of the lower extremity: Definition, epidemiology, and economic and social burdens. Seminars in vascular surgery. 2015 Mar:28(1):3-5. doi: 10.1053/j.semvascsurg.2015.05.002. Epub 2015 May 8
[PubMed PMID: 26358303]
[29]
Margolis DJ, Bilker W, Santanna J, Baumgarten M. Venous leg ulcer: incidence and prevalence in the elderly. Journal of the American Academy of Dermatology. 2002 Mar:46(3):381-6
[PubMed PMID: 11862173]
[30]
Vuylsteke ME, Colman R, Thomis S, Guillaume G, Van Quickenborne D, Staelens I. An Epidemiological Survey of Venous Disease Among General Practitioner Attendees in Different Geographical Regions on the Globe: The Final Results of the Vein Consult Program. Angiology. 2018 Oct:69(9):779-785. doi: 10.1177/0003319718759834. Epub 2018 Feb 26
[PubMed PMID: 29482348]
Level 2 (mid-level) evidence
[31]
Berenguer Pérez M, López-Casanova P, Sarabia Lavín R, González de la Torre H, Verdú-Soriano J. Epidemiology of venous leg ulcers in primary health care: Incidence and prevalence in a health centre-A time series study (2010-2014). International wound journal. 2019 Feb:16(1):256-265. doi: 10.1111/iwj.13026. Epub 2018 Nov 4
[PubMed PMID: 30393963]
[32]
Heit JA, Rooke TW, Silverstein MD, Mohr DN, Lohse CM, Petterson TM, O'Fallon WM, Melton LJ 3rd. Trends in the incidence of venous stasis syndrome and venous ulcer: a 25-year population-based study. Journal of vascular surgery. 2001 May:33(5):1022-7
[PubMed PMID: 11331844]
[33]
Gillespie DL, Writing Group III of the Pacific Vascular Symposium 6, Kistner B, Glass C, Bailey B, Chopra A, Ennis B, Marston B, Masuda E, Moneta G, Nelzen O, Raffetto J, Raju S, Vedantham S, Wright D, Falanga V. Venous ulcer diagnosis, treatment, and prevention of recurrences. Journal of vascular surgery. 2010 Nov:52(5 Suppl):8S-14S. doi: 10.1016/j.jvs.2010.05.068. Epub 2010 Aug 3
[PubMed PMID: 20678885]
[34]
O'Donnell TF Jr, Passman MA, Marston WA, Ennis WJ, Dalsing M, Kistner RL, Lurie F, Henke PK, Gloviczki ML, Eklöf BG, Stoughton J, Raju S, Shortell CK, Raffetto JD, Partsch H, Pounds LC, Cummings ME, Gillespie DL, McLafferty RB, Murad MH, Wakefield TW, Gloviczki P, Society for Vascular Surgery, American Venous Forum. Management of venous leg ulcers: clinical practice guidelines of the Society for Vascular Surgery ® and the American Venous Forum. Journal of vascular surgery. 2014 Aug:60(2 Suppl):3S-59S. doi: 10.1016/j.jvs.2014.04.049. Epub 2014 Jun 25
[PubMed PMID: 24974070]
Level 1 (high-level) evidence
[35]
Di Bella S, Monticelli J, Luzzati R. Evaluation and Management of Lower-Extremity Ulcers. The New England journal of medicine. 2018 Jan 18:378(3):301. doi: 10.1056/NEJMc1715237. Epub
[PubMed PMID: 29345447]
[36]
Kirsner RS, Vivas AC. Lower-extremity ulcers: diagnosis and management. The British journal of dermatology. 2015 Aug:173(2):379-90. doi: 10.1111/bjd.13953. Epub 2015 Aug 8
[PubMed PMID: 26257052]
[37]
Nicolaides AN. The Most Severe Stage of Chronic Venous Disease: An Update on the Management of Patients with Venous Leg Ulcers. Advances in therapy. 2020 Feb:37(Suppl 1):19-24. doi: 10.1007/s12325-020-01219-y. Epub 2020 Jan 22
[PubMed PMID: 31970660]
Level 3 (low-level) evidence
[38]
Singer AJ, Tassiopoulos A, Kirsner RS. Evaluation and Management of Lower-Extremity Ulcers. The New England journal of medicine. 2017 Oct 19:377(16):1559-1567. doi: 10.1056/NEJMra1615243. Epub
[PubMed PMID: 29045216]
[39]
Eklöf B, Rutherford RB, Bergan JJ, Carpentier PH, Gloviczki P, Kistner RL, Meissner MH, Moneta GL, Myers K, Padberg FT, Perrin M, Ruckley CV, Smith PC, Wakefield TW, American Venous Forum International Ad Hoc Committee for Revision of the CEAP Classification. Revision of the CEAP classification for chronic venous disorders: consensus statement. Journal of vascular surgery. 2004 Dec:40(6):1248-52
[PubMed PMID: 15622385]
Level 3 (low-level) evidence
[40]
Kolbach DN, Neumann HA, Prins MH. Definition of the post-thrombotic syndrome, differences between existing classifications. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005 Oct:30(4):404-14
[PubMed PMID: 16009579]
[41]
Vasquez MA, Munschauer CE. Revised venous clinical severity score: a facile measurement of outcomes in venous disease. Phlebology. 2012 Mar:27 Suppl 1():119-29
[PubMed PMID: 22312078]
[42]
Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, Woo K, Romanelli M, Kirsner RS. What's new: Management of venous leg ulcers: Approach to venous leg ulcers. Journal of the American Academy of Dermatology. 2016 Apr:74(4):627-40; quiz 641-2. doi: 10.1016/j.jaad.2014.10.048. Epub
[PubMed PMID: 26979354]
[43]
Andriessen A, Apelqvist J, Mosti G, Partsch H, Gonska C, Abel M. Compression therapy for venous leg ulcers: risk factors for adverse events and complications, contraindications - a review of present guidelines. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017 Sep:31(9):1562-1568. doi: 10.1111/jdv.14390. Epub 2017 Jul 31
[PubMed PMID: 28602045]
[44]
Cavezzi A, Labropoulos N, Partsch H, Ricci S, Caggiati A, Myers K, Nicolaides A, Smith PC. Duplex ultrasound investigation of the veins in chronic venous disease of the lower limbs--UIP consensus document. Part II. Anatomy. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2006 Mar:31(3):288-99
[PubMed PMID: 16230038]
Level 3 (low-level) evidence
[45]
Labropoulos N, Tiongson J, Pryor L, Tassiopoulos AK, Kang SS, Ashraf Mansour M, Baker WH. Definition of venous reflux in lower-extremity veins. Journal of vascular surgery. 2003 Oct:38(4):793-8
[PubMed PMID: 14560232]
[46]
van Bemmelen PS, van Ramshorst B, Eikelboom BC. Photoplethysmography reexamined: lack of correlation with duplex scanning. Surgery. 1992 Sep:112(3):544-8
[PubMed PMID: 1519171]
[47]
Neglén P, Raju S. Intravascular ultrasound scan evaluation of the obstructed vein. Journal of vascular surgery. 2002 Apr:35(4):694-700
[PubMed PMID: 11932665]
[48]
Lurie F, Bittar S, Kasper G. Optimal Compression Therapy and Wound Care for Venous Ulcers. The Surgical clinics of North America. 2018 Apr:98(2):349-360. doi: 10.1016/j.suc.2017.11.006. Epub 2017 Dec 18
[PubMed PMID: 29502776]
[49]
Alavi A, Sibbald RG, Phillips TJ, Miller OF, Margolis DJ, Marston W, Woo K, Romanelli M, Kirsner RS. What's new: Management of venous leg ulcers: Treating venous leg ulcers. Journal of the American Academy of Dermatology. 2016 Apr:74(4):643-64; quiz 665-6. doi: 10.1016/j.jaad.2015.03.059. Epub
[PubMed PMID: 26979355]
[50]
O'Meara S, Cullum N, Nelson EA, Dumville JC. Compression for venous leg ulcers. The Cochrane database of systematic reviews. 2012 Nov 14:11(11):CD000265. doi: 10.1002/14651858.CD000265.pub3. Epub 2012 Nov 14
[PubMed PMID: 23152202]
Level 1 (high-level) evidence
[51]
Nelson EA, Hillman A, Thomas K. Intermittent pneumatic compression for treating venous leg ulcers. The Cochrane database of systematic reviews. 2014 May 12:2014(5):CD001899. doi: 10.1002/14651858.CD001899.pub4. Epub 2014 May 12
[PubMed PMID: 24820100]
Level 1 (high-level) evidence
[52]
Szolnoky G, Tuczai M, Macdonald JM, Dosa-Racz E, Barsony K, Balogh M, Szabad G, Dobozy A, Kemeny L. Adjunctive role of manual lymph drainage in the healing of venous ulcers: A comparative pilot study. Lymphology. 2018:51(4):148-159
[PubMed PMID: 31119905]
Level 2 (mid-level) evidence
[53]
Cooper B, Bachoo P. Extracorporeal shock wave therapy for the healing and management of venous leg ulcers. The Cochrane database of systematic reviews. 2018 Jun 11:6(6):CD011842. doi: 10.1002/14651858.CD011842.pub2. Epub 2018 Jun 11
[PubMed PMID: 29889978]
Level 1 (high-level) evidence
[54]
Nair B. Venous leg ulcer: Systemic therapy. Indian dermatology online journal. 2014 Jul:5(3):374-7. doi: 10.4103/2229-5178.137821. Epub
[PubMed PMID: 25165678]
[55]
Jull AB, Waters J, Arroll B. Oral pentoxifylline for treatment of venous leg ulcers. The Cochrane database of systematic reviews. 2000:(2):CD001733
[PubMed PMID: 10796661]
Level 1 (high-level) evidence
[56]
Jull A, Waters J, Arroll B. Pentoxifylline for treatment of venous leg ulcers: a systematic review. Lancet (London, England). 2002 May 4:359(9317):1550-4
[PubMed PMID: 12047963]
Level 1 (high-level) evidence
[57]
Smith PC. Daflon 500 mg and venous leg ulcer: new results from a meta-analysis. Angiology. 2005 Sep-Oct:56 Suppl 1():S33-9
[PubMed PMID: 16193225]
Level 1 (high-level) evidence
[58]
Coleridge-Smith P, Lok C, Ramelet AA. Venous leg ulcer: a meta-analysis of adjunctive therapy with micronized purified flavonoid fraction. European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2005 Aug:30(2):198-208
[PubMed PMID: 15936227]
Level 1 (high-level) evidence
[59]
de Oliveira Carvalho PE, Magolbo NG, De Aquino RF, Weller CD. Oral aspirin for treating venous leg ulcers. The Cochrane database of systematic reviews. 2016 Feb 18:2(2):CD009432. doi: 10.1002/14651858.CD009432.pub2. Epub 2016 Feb 18
[PubMed PMID: 26889740]
Level 1 (high-level) evidence
[60]
Norman G, Westby MJ, Rithalia AD, Stubbs N, Soares MO, Dumville JC. Dressings and topical agents for treating venous leg ulcers. The Cochrane database of systematic reviews. 2018 Jun 15:6(6):CD012583. doi: 10.1002/14651858.CD012583.pub2. Epub 2018 Jun 15
[PubMed PMID: 29906322]
Level 1 (high-level) evidence
[61]
Jones JE, Nelson EA, Al-Hity A. Skin grafting for venous leg ulcers. The Cochrane database of systematic reviews. 2013 Jan 31:2013(1):CD001737. doi: 10.1002/14651858.CD001737.pub4. Epub 2013 Jan 31
[PubMed PMID: 23440784]
Level 1 (high-level) evidence
[62]
Montminy ML, Jayaraj A, Raju S. A systematic review of the efficacy and limitations of venous intervention in stasis ulceration. Journal of vascular surgery. Venous and lymphatic disorders. 2018 May:6(3):376-398.e1. doi: 10.1016/j.jvsv.2017.11.007. Epub 2018 Feb 13
[PubMed PMID: 29452957]
Level 1 (high-level) evidence
[63]
Gohel MS, Heatley F, Liu X, Bradbury A, Bulbulia R, Cullum N, Epstein DM, Nyamekye I, Poskitt KR, Renton S, Warwick J, Davies AH, EVRA Trial Investigators. A Randomized Trial of Early Endovenous Ablation in Venous Ulceration. The New England journal of medicine. 2018 May 31:378(22):2105-2114. doi: 10.1056/NEJMoa1801214. Epub 2018 Apr 24
[PubMed PMID: 29688123]
Level 1 (high-level) evidence
[64]
Stansal A, Khayat K, Duchatelle V, Tella E, Gautier V, Sfeir D, Attal R, Lazareth I, Priollet P. [When to ask for a skin biopsy in a patient with leg ulcer? Retrospective study of 143 consecutive biopsies]. Journal de medecine vasculaire. 2018 Feb:43(1):4-9. doi: 10.1016/j.jdmv.2017.10.003. Epub 2017 Nov 21
[PubMed PMID: 29425540]
Level 2 (mid-level) evidence
[65]
Tchanque-Fossuo CN, Millsop JW, Johnson MA, Dahle SE, Isseroff RR. Ulcerated Basal Cell Carcinomas Masquerading as Venous Leg Ulcers. Advances in skin & wound care. 2018 Mar:31(3):130-134. doi: 10.1097/01.ASW.0000530068.44631.dc. Epub
[PubMed PMID: 29438147]
Level 3 (low-level) evidence
[66]
Senet P, Combemale P, Debure C, Baudot N, Machet L, Aout M, Vicaut E, Lok C, Angio-Dermatology Group Of The French Society Of Dermatology. Malignancy and chronic leg ulcers: the value of systematic wound biopsies: a prospective, multicenter, cross-sectional study. Archives of dermatology. 2012 Jun:148(6):704-8. doi: 10.1001/archdermatol.2011.3362. Epub
[PubMed PMID: 22772403]
Level 2 (mid-level) evidence
[67]
Margolis DJ, Allen-Taylor L, Hoffstad O, Berlin JA. The accuracy of venous leg ulcer prognostic models in a wound care system. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2004 Mar-Apr:12(2):163-8
[PubMed PMID: 15086767]
[68]
Melikian R, O'Donnell TF Jr, Suarez L, Iafrati MD. Risk factors associated with the venous leg ulcer that fails to heal after 1 year of treatment. Journal of vascular surgery. Venous and lymphatic disorders. 2019 Jan:7(1):98-105. doi: 10.1016/j.jvsv.2018.07.014. Epub 2018 Oct 24
[PubMed PMID: 30558732]
[69]
Parker CN, Finlayson KJ, Shuter P, Edwards HE. Risk factors for delayed healing in venous leg ulcers: a review of the literature. International journal of clinical practice. 2015 Sep:69(9):967-77. doi: 10.1111/ijcp.12635. Epub 2015 Apr 1
[PubMed PMID: 25831965]
[70]
Kantor J, Margolis DJ. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. The British journal of dermatology. 2000 May:142(5):960-4
[PubMed PMID: 10809855]
[71]
Gelfand JM, Hoffstad O, Margolis DJ. Surrogate endpoints for the treatment of venous leg ulcers. The Journal of investigative dermatology. 2002 Dec:119(6):1420-5
[PubMed PMID: 12485449]
[72]
Gorin DR, Cordts PR, LaMorte WW, Manzoian JO. The influence of wound geometry on the measurement of wound healing rates in clinical trials. Journal of vascular surgery. 1996 Mar:23(3):524-8
[PubMed PMID: 8601898]
[73]
Gilman T. A multicentre study of percentage change in venous leg ulcer area as a prognostic index of healing at 24 weeks. The British journal of dermatology. 2003 Oct:149(4):896-7; author reply 897-8
[PubMed PMID: 14616394]
[74]
Mauck KF, Asi N, Elraiyah TA, Undavalli C, Nabhan M, Altayar O, Sonbol MB, Prokop LJ, Murad MH. Comparative systematic review and meta-analysis of compression modalities for the promotion of venous ulcer healing and reducing ulcer recurrence. Journal of vascular surgery. 2014 Aug:60(2 Suppl):71S-90S.e1-2. doi: 10.1016/j.jvs.2014.04.060. Epub 2014 May 28
[PubMed PMID: 24877851]
Level 2 (mid-level) evidence
[75]
Atkin L, Martin R. An audience survey of practice relating to pain in the management of chronic venous leg ulcers. British journal of community nursing. 2020 Dec 1:25(Sup12):S20-S24. doi: 10.12968/bjcn.2020.25.Sup12.S20. Epub
[PubMed PMID: 33300846]
Level 3 (low-level) evidence
[76]
Abbade LP, Lastória S, de Almeida Rollo H, Stolf HO. A sociodemographic, clinical study of patients with venous ulcer. International journal of dermatology. 2005 Dec:44(12):989-92
[PubMed PMID: 16409260]
[77]
Jindal R, Dekiwadia DB, Krishna PR, Khanna AK, Patel MD, Padaria S, Varghese R. Evidence-Based Clinical Practice Points for the Management of Venous Ulcers. The Indian journal of surgery. 2018 Apr:80(2):171-182. doi: 10.1007/s12262-018-1726-3. Epub 2018 Jan 27
[PubMed PMID: 29915484]
[78]
Couch KS, Corbett L, Gould L, Girolami S, Bolton L. The International Consolidated Venous Ulcer Guideline Update 2015: Process Improvement, Evidence Analysis, and Future Goals. Ostomy/wound management. 2017 May:63(5):42-46
[PubMed PMID: 28570248]
[79]
O'Donnell TF Jr, Balk EM. The need for an Intersociety Consensus Guideline for venous ulcer. Journal of vascular surgery. 2011 Dec:54(6 Suppl):83S-90S. doi: 10.1016/j.jvs.2011.06.001. Epub 2011 Sep 9
[PubMed PMID: 21908148]
Level 3 (low-level) evidence
[80]
Tan MKH, Luo R, Onida S, Maccatrozzo S, Davies AH. Venous Leg Ulcer Clinical Practice Guidelines: What is AGREEd? European journal of vascular and endovascular surgery : the official journal of the European Society for Vascular Surgery. 2019 Jan:57(1):121-129. doi: 10.1016/j.ejvs.2018.08.043. Epub 2018 Oct 2
[PubMed PMID: 30287207]
Level 1 (high-level) evidence
[81]
Mooij MC, Huisman LC. Chronic leg ulcer: does a patient always get a correct diagnosis and adequate treatment? Phlebology. 2016 Mar:31(1 Suppl):68-73. doi: 10.1177/0268355516632436. Epub
[PubMed PMID: 26916772]
[82]
Flanagan M, Rotchell L, Fletcher J, Schofield J. Community nurses', home carers' and patients' perceptions of factors affecting venous leg ulcer recurrence and management of services. Journal of nursing management. 2001 May:9(3):153-9
[PubMed PMID: 11879462]
[83]
Jull A, Slark J, Parsons J. Prescribed Exercise With Compression vs Compression Alone in Treating Patients With Venous Leg Ulcers: A Systematic Review and Meta-analysis. JAMA dermatology. 2018 Nov 1:154(11):1304-1311. doi: 10.1001/jamadermatol.2018.3281. Epub
[PubMed PMID: 30285080]
Level 1 (high-level) evidence
[84]
Barber GA, Weller CD, Gibson SJ. Effects and associations of nutrition in patients with venous leg ulcers: A systematic review. Journal of advanced nursing. 2018 Apr:74(4):774-787. doi: 10.1111/jan.13474. Epub 2017 Nov 9
[PubMed PMID: 28985441]
Level 1 (high-level) evidence