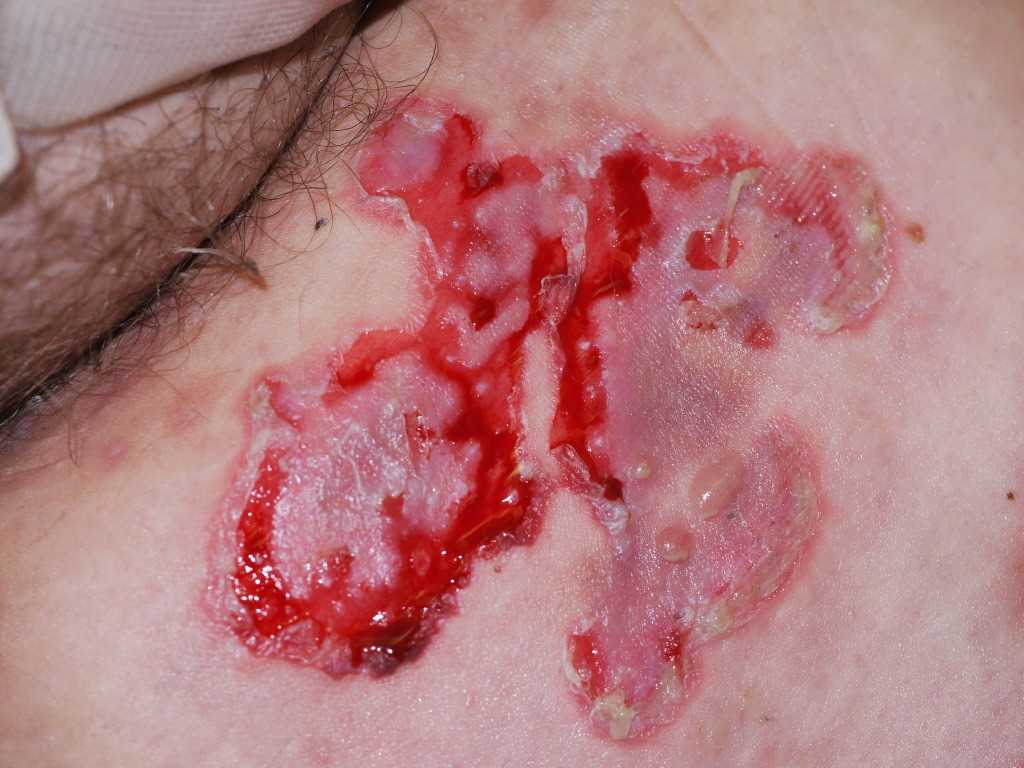
[1]
Patel F, Wilken R, Patel FB, Sultani H, Bustos I, Duong C, Zone JJ, Raychaudhuri SP, Maverakis E. Pathophysiology of Autoimmune Bullous Diseases: Nature Versus Nurture. Indian journal of dermatology. 2017 May-Jun:62(3):262-267. doi: 10.4103/0019-5154.159620. Epub
[PubMed PMID: 28584368]
[3]
Jain VR, Mahajan RS, Rathi SS, Biyani VV, Ninama KR, Marfatia YS. Oral Mucosal Lesions - A Study of 369 Cases. Indian dermatology online journal. 2023 Mar-Apr:14(2):213-220. doi: 10.4103/idoj.idoj_203_22. Epub 2023 Mar 3
[PubMed PMID: 37089853]
Level 3 (low-level) evidence
[4]
Zhu Y, Su J, Zhang P, Deng M, Wu R, Liu Y, Su Y, Li S. The dysregulation of circulating innate lymphoid cells is related to autoantibodies in pemphigus vulgaris. International immunopharmacology. 2023 Apr:117():109921. doi: 10.1016/j.intimp.2023.109921. Epub 2023 Feb 24
[PubMed PMID: 36841156]
[5]
Drenovska K, Ivanova M, Vassileva S, Shahid MA, Naumova E. Association of specific HLA alleles and haplotypes with pemphigus vulgaris in the Bulgarian population. Frontiers in immunology. 2022:13():901386. doi: 10.3389/fimmu.2022.901386. Epub 2022 Aug 2
[PubMed PMID: 35983062]
[6]
Salviano-Silva A, Farias TDJ, Bumiller-Bini V, Castro MS, Lobo-Alves SC, Busch H, Pföhler C, Worm M, Goebeler M, van Beek N, Franke A, Wittig M, Zillikens D, de Almeida RC, Hundt JE, Boldt ABW, Ibrahim S, Augusto DG, Petzl-Erler ML, Schmidt E, Malheiros D. Genetic variability of immune-related lncRNAs: polymorphisms in LINC-PINT and LY86-AS1 are associated with pemphigus foliaceus susceptibility. Experimental dermatology. 2021 Jun:30(6):831-840. doi: 10.1111/exd.14275. Epub 2021 Jan 15
[PubMed PMID: 33394553]
[7]
Baker J, Seiffert-Sinha K, Sinha AA. Patient genetics shape the autoimmune response in the blistering skin disease pemphigus vulgaris. Frontiers in immunology. 2022:13():1064073. doi: 10.3389/fimmu.2022.1064073. Epub 2023 Jan 10
[PubMed PMID: 36703961]
[8]
Firooz A, Mazhar A, Ahmed AR. Prevalence of autoimmune diseases in the family members of patients with pemphigus vulgaris. Journal of the American Academy of Dermatology. 1994 Sep:31(3 Pt 1):434-7
[PubMed PMID: 8077468]
[9]
Szafer F, Brautbar C, Tzfoni E, Frankel G, Sherman L, Cohen I, Hacham-Zadeh S, Aberer W, Tappeiner G, Holubar K. Detection of disease-specific restriction fragment length polymorphisms in pemphigus vulgaris linked to the DQw1 and DQw3 alleles of the HLA-D region. Proceedings of the National Academy of Sciences of the United States of America. 1987 Sep:84(18):6542-5
[PubMed PMID: 2888115]
[10]
Lombardi ML, Mercuro O, Ruocco V, Lo Schiavo A, Lombari V, Guerrera V, Pirozzi G, Manzo C. Common human leukocyte antigen alleles in pemphigus vulgaris and pemphigus foliaceus Italian patients. The Journal of investigative dermatology. 1999 Jul:113(1):107-10
[PubMed PMID: 10417627]
[11]
Tavakolpour S. Pemphigus trigger factors: special focus on pemphigus vulgaris and pemphigus foliaceus. Archives of dermatological research. 2018 Mar:310(2):95-106. doi: 10.1007/s00403-017-1790-8. Epub 2017 Nov 6
[PubMed PMID: 29110080]
[12]
Crocker C. Radiation-induced pemphigus in a patient with an invasive ductal carcinoma of the breast: a case report. Oxford medical case reports. 2020 Jan:2020(1):omaa001. doi: 10.1093/omcr/omaa001. Epub 2020 Jan 31
[PubMed PMID: 32038873]
Level 3 (low-level) evidence
[13]
Brenner S, Goldberg I. Drug-induced pemphigus. Clinics in dermatology. 2011 Jul-Aug:29(4):455-7. doi: 10.1016/j.clindermatol.2011.01.016. Epub
[PubMed PMID: 21679874]
[14]
Feng S, Zhou W, Zhang J, Jin P. Analysis of 6 cases of drug-induced pemphigus. European journal of dermatology : EJD. 2011 Sep-Oct:21(5):696-9. doi: 10.1684/ejd.2011.1428. Epub
[PubMed PMID: 21697060]
Level 3 (low-level) evidence
[15]
Ruocco V, Ruocco E, Lo Schiavo A, Brunetti G, Guerrera LP, Wolf R. Pemphigus: etiology, pathogenesis, and inducing or triggering factors: facts and controversies. Clinics in dermatology. 2013 Jul-Aug:31(4):374-381. doi: 10.1016/j.clindermatol.2013.01.004. Epub
[PubMed PMID: 23806154]
[16]
Celere BS, Vernal S, Brochado MJF, Segura-Muñoz SI, Roselino AM. Geographical foci and epidemiological changes of pemphigus vulgaris in four decades in Southeastern Brazil. International journal of dermatology. 2017 Dec:56(12):1494-1496. doi: 10.1111/ijd.13714. Epub 2017 Jul 24
[PubMed PMID: 28737218]
Level 2 (mid-level) evidence
[17]
Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris--incidence and mortality in the UK: population based cohort study. BMJ (Clinical research ed.). 2008 Jul 9:337(7662):a180. doi: 10.1136/bmj.a180. Epub 2008 Jul 9
[PubMed PMID: 18614511]
[18]
Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 1: Clinical manifestations. Journal der Deutschen Dermatologischen Gesellschaft = Journal of the German Society of Dermatology : JDDG. 2011 Oct:9(10):844-56; quiz 857. doi: 10.1111/j.1610-0387.2011.07793.x. Epub
[PubMed PMID: 21955378]
[19]
Porro AM, Seque CA, Ferreira MCC, Enokihara MMSES. Pemphigus vulgaris. Anais brasileiros de dermatologia. 2019 Jul 29:94(3):264-278. doi: 10.1590/abd1806-4841.20199011. Epub 2019 Jul 29
[PubMed PMID: 31365654]
[20]
Kridin K. Pemphigus group: overview, epidemiology, mortality, and comorbidities. Immunologic research. 2018 Apr:66(2):255-270. doi: 10.1007/s12026-018-8986-7. Epub
[PubMed PMID: 29479654]
Level 3 (low-level) evidence
[21]
Vodo D, Sarig O, Sprecher E. The Genetics of Pemphigus Vulgaris. Frontiers in medicine. 2018:5():226. doi: 10.3389/fmed.2018.00226. Epub 2018 Aug 14
[PubMed PMID: 30155467]
[22]
Guptill JT, Sleasman JW, Steeland S, Sips M, Gelinas D, de Haard H, Azar A, Winthrop KL. Effect of FcRn antagonism on protective antibodies and to vaccines in IgG-mediated autoimmune diseases pemphigus and generalised myasthenia gravis. Autoimmunity. 2022 Dec:55(8):620-631. doi: 10.1080/08916934.2022.2104261. Epub 2022 Aug 29
[PubMed PMID: 36036539]
[23]
Seo JW, Park J, Lee J, Kim MY, Choi HJ, Jeong HJ, Lee JW, Jung SY, Kim WK. A case of pemphigus vulgaris associated with ulcerative colitis. Intestinal research. 2018 Jan:16(1):147-150. doi: 10.5217/ir.2018.16.1.147. Epub 2018 Jan 18
[PubMed PMID: 29422810]
Level 3 (low-level) evidence
[24]
Alshami ML, Aswad F, Abdullah B. Desmogleins 1, 3, and E-cadherin immunohistochemical expression within mucocutaneous pemphigus vulgaris. The Pan African medical journal. 2022:42():186. doi: 10.11604/pamj.2022.42.186.35429. Epub 2022 Jul 7
[PubMed PMID: 36212929]
[25]
Ishii N. Significance of anti-desmocollin autoantibodies in pemphigus. The Journal of dermatology. 2023 Feb:50(2):132-139. doi: 10.1111/1346-8138.16660. Epub 2022 Dec 28
[PubMed PMID: 36578135]
[26]
Mihai S, Sitaru C. Immunopathology and molecular diagnosis of autoimmune bullous diseases. Journal of cellular and molecular medicine. 2007 May-Jun:11(3):462-81
[PubMed PMID: 17521373]
[27]
Hertl M, Eming R, Veldman C. T cell control in autoimmune bullous skin disorders. The Journal of clinical investigation. 2006 May:116(5):1159-66
[PubMed PMID: 16670756]
[28]
Sinha AA, Sajda T. The Evolving Story of Autoantibodies in Pemphigus Vulgaris: Development of the "Super Compensation Hypothesis". Frontiers in medicine. 2018:5():218. doi: 10.3389/fmed.2018.00218. Epub 2018 Aug 14
[PubMed PMID: 30155465]
[29]
Sitaru C, Zillikens D. Mechanisms of blister induction by autoantibodies. Experimental dermatology. 2005 Dec:14(12):861-75
[PubMed PMID: 16274453]
[30]
Hammers CM, Stanley JR. Mechanisms of Disease: Pemphigus and Bullous Pemphigoid. Annual review of pathology. 2016 May 23:11():175-97. doi: 10.1146/annurev-pathol-012615-044313. Epub 2016 Feb 22
[PubMed PMID: 26907530]
[31]
Amagai M, Tsunoda K, Zillikens D, Nagai T, Nishikawa T. The clinical phenotype of pemphigus is defined by the anti-desmoglein autoantibody profile. Journal of the American Academy of Dermatology. 1999 Feb:40(2 Pt 1):167-70
[PubMed PMID: 10025740]
[32]
Didona D, Maglie R, Eming R, Hertl M. Pemphigus: Current and Future Therapeutic Strategies. Frontiers in immunology. 2019:10():1418. doi: 10.3389/fimmu.2019.01418. Epub 2019 Jun 25
[PubMed PMID: 31293582]
[33]
Amagai M, Karpati S, Prussick R, Klaus-Kovtun V, Stanley JR. Autoantibodies against the amino-terminal cadherin-like binding domain of pemphigus vulgaris antigen are pathogenic. The Journal of clinical investigation. 1992 Sep:90(3):919-26
[PubMed PMID: 1522242]
[34]
Hartmann V, Hariton WV, Rahimi S, Hammers CM, Ludwig RJ, Müller EJ, Hundt JE. The human skin organ culture model as an optimal complementary tool for murine pemphigus models. Laboratory animals. 2023 Aug:57(4):381-395. doi: 10.1177/00236772221145647. Epub 2023 Jan 16
[PubMed PMID: 36647613]
Level 3 (low-level) evidence
[35]
Boch K, Dräger S, Zillikens D, Hudemann C, Hammers CM, Patzelt S, Schmidt E, Langan EA, Eming R, Ludwig RJ, Bieber K. Immunization with desmoglein 3 induces non-pathogenic autoantibodies in mice. PloS one. 2021:16(11):e0259586. doi: 10.1371/journal.pone.0259586. Epub 2021 Nov 3
[PubMed PMID: 34731225]
[36]
Egu DT, Schmitt T, Waschke J. Mechanisms Causing Acantholysis in Pemphigus-Lessons from Human Skin. Frontiers in immunology. 2022:13():884067. doi: 10.3389/fimmu.2022.884067. Epub 2022 May 20
[PubMed PMID: 35720332]
[37]
Kawamura T, Muramatsu K, Orita A, Mai Y, Sugai T, Haga N, Fujimura Y, Miyauchi T, Izumi K, Koga H, Ishii N, Ujiie H. Two cases of Hallopeau-type pemphigus vegetans with anti-desmoglein 1 and anti-desmocollin 3 antibodies without mucosal involvement. Journal of the European Academy of Dermatology and Venereology : JEADV. 2023 Apr:37(4):e508-e510. doi: 10.1111/jdv.18704. Epub 2022 Nov 7
[PubMed PMID: 36305887]
Level 3 (low-level) evidence
[38]
Jindal A, Rao C, Pai SB, Rao R. Utility of oral mucosa as a substrate for the serodiagnosis of pemphigus: A descriptive analysis. Indian journal of dermatology, venereology and leprology. 2022 Mar-Apr:88(2):156-161. doi: 10.25259/IJDVL_469_20. Epub
[PubMed PMID: 34491669]
[39]
Ishii K, Yoshida K, Stanley JR, Yamagami J, Amagai M, Ishiko A. Pemphigus Vulgaris and Foliaceus IgG Autoantibodies Directly Block Heterophilic Transinteraction between Desmoglein and Desmocollin. The Journal of investigative dermatology. 2020 Oct:140(10):1919-1926.e7. doi: 10.1016/j.jid.2020.02.010. Epub 2020 Mar 3
[PubMed PMID: 32142800]
[40]
Schmitt T, Waschke J. Autoantibody-Specific Signalling in Pemphigus. Frontiers in medicine. 2021:8():701809. doi: 10.3389/fmed.2021.701809. Epub 2021 Aug 9
[PubMed PMID: 34434944]
[41]
Oktarina DA, van der Wier G, Diercks GF, Jonkman MF, Pas HH. IgG-induced clustering of desmogleins 1 and 3 in skin of patients with pemphigus fits with the desmoglein nonassembly depletion hypothesis. The British journal of dermatology. 2011 Sep:165(3):552-62. doi: 10.1111/j.1365-2133.2011.10463.x. Epub
[PubMed PMID: 21692763]
[42]
Sielski L, Baker J, DePasquale MC, Attwood K, Seiffert-Sinha K, Sinha AA. Desmoglein compensation hypothesis fidelity assessment in Pemphigus. Frontiers in immunology. 2022:13():969278. doi: 10.3389/fimmu.2022.969278. Epub 2022 Sep 23
[PubMed PMID: 36211362]
[43]
Garg R, Bhojani K. Non infective bullous lesions: a diagnostic challenge in a minimally equipped centre- based solely on microscopic findings. African health sciences. 2020 Jun:20(2):885-890. doi: 10.4314/ahs.v20i2.42. Epub
[PubMed PMID: 33163055]
[44]
Papara C, Danescu S, Rogojan L, Leucuta DC, Candrea E, Zillikens D, Baican A. Lymphocyte-predominant lesional inflammatory infiltrates of the skin are associated with mucosal-dominant phenotype in pemphigus. Journal of cutaneous pathology. 2023 Aug:50(8):754-762. doi: 10.1111/cup.14395. Epub 2023 Feb 14
[PubMed PMID: 36680509]
[45]
Gottesman SP, Abedi SM, Rosen JR, Gottlieb GJ. Hailey-Hailey-Like Pattern of Acantholysis on the Scalp Should Raise the Possibility of Incipient Pemphigus Vulgaris. The American Journal of dermatopathology. 2019 Apr:41(4):286-288. doi: 10.1097/DAD.0000000000001298. Epub
[PubMed PMID: 30640761]
[46]
See SHC, Peternel S, Adams D, North JP. Distinguishing histopathologic features of acantholytic dermatoses and the pattern of acantholytic hypergranulosis. Journal of cutaneous pathology. 2019 Jan:46(1):6-15. doi: 10.1111/cup.13356. Epub 2018 Nov 5
[PubMed PMID: 30203619]
[47]
Ng JKM, Cheung CM, Choi PCL, Ip EC, Li JJX, Chan AWS. A rare presentation of pemphigus vegetans as an isolated vegetative lesion-Review of histopathological clues and treatment effects in multiple biopsy specimens. Journal of cutaneous pathology. 2023 Mar:50(3):201-208. doi: 10.1111/cup.14375. Epub 2023 Jan 4
[PubMed PMID: 36502456]
[48]
Jabri H, Hali F, Elkaroini D, Mahdaoui S, Alatawna H, Chiheb S. Pemphigus vegetans-Neumann variant of the vulva. International journal of dermatology. 2023 Jun:62(6):841-842. doi: 10.1111/ijd.16702. Epub 2023 Apr 26
[PubMed PMID: 37098705]
[49]
Davarmanesh M, Zahed M, Sookhakian A, Jehbez S. Oral Pemphigus Vulgaris Treatment with Corticosteroids and Azathioprine: A Long-Term Study in Shiraz, Iran. Evidence-based complementary and alternative medicine : eCAM. 2022:2022():7583691. doi: 10.1155/2022/7583691. Epub 2022 Sep 17
[PubMed PMID: 36164397]
[50]
Korbl JD, Brusch A, Lucas M, von Nida J, Wood BA, Leecy TN, Harvey NT. A case of severe cutaneous and mucosal erosions. Clinical and experimental dermatology. 2020 Aug:45(6):780-782. doi: 10.1111/ced.14233. Epub 2020 May 14
[PubMed PMID: 32410330]
Level 3 (low-level) evidence
[51]
Yang M, Wu H, Zhao M, Long H, Lu Q. Vitamin D status in patients with autoimmune bullous dermatoses: a meta-analysis. The Journal of dermatological treatment. 2022 May:33(3):1356-1367. doi: 10.1080/09546634.2020.1810606. Epub 2020 Aug 26
[PubMed PMID: 32799714]
Level 1 (high-level) evidence
[52]
Lamichhane R, Chaudhary S. Delayed Diagnosis of Pemphigus Vulgaris Initially Presenting as an Oral Ulcer: A Case Report. JNMA; journal of the Nepal Medical Association. 2022 Jul 1:60(251):641-643. doi: 10.31729/jnma.7594. Epub 2022 Jul 1
[PubMed PMID: 36705190]
Level 3 (low-level) evidence
[53]
Silva SC, Nasser R, Payne AS, Stoopler ET. Pemphigus Vulgaris. The Journal of emergency medicine. 2019 Jan:56(1):102-104. doi: 10.1016/j.jemermed.2018.10.028. Epub 2018 Nov 30
[PubMed PMID: 30503724]
[54]
Kavala M, Topaloğlu Demir F, Zindanci I, Can B, Turkoğlu Z, Zemheri E, Cam OH, Teksen A. Genital involvement in pemphigus vulgaris (PV): correlation with clinical and cervicovaginal Pap smear findings. Journal of the American Academy of Dermatology. 2015 Oct:73(4):655-9. doi: 10.1016/j.jaad.2015.06.057. Epub 2015 Jul 17
[PubMed PMID: 26194705]
[55]
Munhoz de Paula Alves Coelho K, Stall J, Henrique Condeixa de França P, Cristina de Carvalho Tavares L, Stefanello Bublitz G, Loos B, Carvalho Costa L, Fronza Júnior H. Pemphigus vulgaris of the cervix: diagnostic difficulties associated with the Pap test. Diagnostic cytopathology. 2015 Aug:43(8):635-7. doi: 10.1002/dc.23269. Epub 2015 Feb 26
[PubMed PMID: 25728997]
[56]
Akhyani M, Chams-Davatchi C, Naraghi Z, Daneshpazhooh M, Toosi S, Asgari M, Malekhami F. Cervicovaginal involvement in pemphigus vulgaris: a clinical study of 77 cases. The British journal of dermatology. 2008 Mar:158(3):478-82
[PubMed PMID: 18070212]
Level 3 (low-level) evidence
[57]
Hertl M, Veldman C. Pemphigus--paradigm of autoantibody-mediated autoimmunity. Skin pharmacology and applied skin physiology. 2001 Nov-Dec:14(6):408-18
[PubMed PMID: 11598441]
[58]
Xie D, Bilgic A, Abu Alrub N, Dicle Ö, Murrell DF. Clinical manifestations of alopecia in autoimmune blistering diseases: A cross-sectional study. JAAD international. 2023 Mar:10():6-13. doi: 10.1016/j.jdin.2022.08.025. Epub 2022 Oct 11
[PubMed PMID: 36387063]
Level 2 (mid-level) evidence
[59]
Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet (London, England). 2019 Sep 7:394(10201):882-894. doi: 10.1016/S0140-6736(19)31778-7. Epub
[PubMed PMID: 31498102]
[60]
Helm M, Helm LA, Clebak KT, Foulke G. Autoimmune Skin Conditions: Autoimmune Blistering Disease. FP essentials. 2023 Mar:526():13-17
[PubMed PMID: 36913658]
[61]
Asokan S, Rai R, Boppe A, Umamaheswari G. Evaluation of the Role of Oral Mucosal Direct Immunofluorescence and Salivary Desmoglein 1 and 3 Enzyme-Linked Immunosorbent Assay in Patients With Oral Mucosal Pemphigus. Indian dermatology online journal. 2022 Sep-Oct:13(5):617-619. doi: 10.4103/idoj.idoj_44_22. Epub 2022 Sep 5
[PubMed PMID: 36304655]
[62]
Pohla-Gubo G, Hintner H. Direct and indirect immunofluorescence for the diagnosis of bullous autoimmune diseases. Dermatologic clinics. 2011 Jul:29(3):365-72, vii. doi: 10.1016/j.det.2011.03.001. Epub
[PubMed PMID: 21605801]
[63]
Hocke J, Krauth J, Krause C, Gerlach S, Warnemünde N, Affeldt K, van Beek N, Schmidt E, Voigt J. Computer-aided classification of indirect immunofluorescence patterns on esophagus and split skin for the detection of autoimmune dermatoses. Frontiers in immunology. 2023:14():1111172. doi: 10.3389/fimmu.2023.1111172. Epub 2023 Feb 28
[PubMed PMID: 36926325]
[64]
Harman KE, Brown D, Exton LS, Groves RW, Hampton PJ, Mohd Mustapa MF, Setterfield JF, Yesudian PD. British Association of Dermatologists' guidelines for the management of pemphigus vulgaris 2017. The British journal of dermatology. 2017 Nov:177(5):1170-1201. doi: 10.1111/bjd.15930. Epub
[PubMed PMID: 29192996]
[65]
Hertl M, Jedlickova H, Karpati S, Marinovic B, Uzun S, Yayli S, Mimouni D, Borradori L, Feliciani C, Ioannides D, Joly P, Kowalewski C, Zambruno G, Zillikens D, Jonkman MF. Pemphigus. S2 Guideline for diagnosis and treatment--guided by the European Dermatology Forum (EDF) in cooperation with the European Academy of Dermatology and Venereology (EADV). Journal of the European Academy of Dermatology and Venereology : JEADV. 2015 Mar:29(3):405-14. doi: 10.1111/jdv.12772. Epub 2014 Oct 22
[PubMed PMID: 25338479]
[66]
Kridin K. Emerging treatment options for the management of pemphigus vulgaris. Therapeutics and clinical risk management. 2018:14():757-778. doi: 10.2147/TCRM.S142471. Epub 2018 Apr 27
[PubMed PMID: 29740210]
[69]
Davis A, Dickson AL, Daniel LL, Nepal P, Zanussi J, Miller-Fleming TW, Straub PS, Wei WQ, Liu G, Cox NJ, Hung AM, Feng Q, Stein CM, Chung CP. Association Between Genetically Predicted Expression of TPMT and Azathioprine Adverse Events. Research square. 2023 Jan 13:():. pii: rs.3.rs-2444787. doi: 10.21203/rs.3.rs-2444787/v1. Epub 2023 Jan 13
[PubMed PMID: 36711487]
[70]
Murrell DF, Peña S, Joly P, Marinovic B, Hashimoto T, Diaz LA, Sinha AA, Payne AS, Daneshpazhooh M, Eming R, Jonkman MF, Mimouni D, Borradori L, Kim SC, Yamagami J, Lehman JS, Saleh MA, Culton DA, Czernik A, Zone JJ, Fivenson D, Ujiie H, Wozniak K, Akman-Karakaş A, Bernard P, Korman NJ, Caux F, Drenovska K, Prost-Squarcioni C, Vassileva S, Feldman RJ, Cardones AR, Bauer J, Ioannides D, Jedlickova H, Palisson F, Patsatsi A, Uzun S, Yayli S, Zillikens D, Amagai M, Hertl M, Schmidt E, Aoki V, Grando SA, Shimizu H, Baum S, Cianchini G, Feliciani C, Iranzo P, Mascaró JM Jr, Kowalewski C, Hall R, Groves R, Harman KE, Marinkovich MP, Maverakis E, Werth VP. Diagnosis and management of pemphigus: Recommendations of an international panel of experts. Journal of the American Academy of Dermatology. 2020 Mar:82(3):575-585.e1. doi: 10.1016/j.jaad.2018.02.021. Epub 2018 Feb 10
[PubMed PMID: 29438767]
[71]
Garcia N, Patel OU, Graham L. Novel pain management therapies for patients with pemphigus. International journal of dermatology. 2023 Apr:62(4):575-578. doi: 10.1111/ijd.16331. Epub 2022 Jul 4
[PubMed PMID: 35781697]
[72]
Sigmund AM, Winkler M, Engelmayer S, Kugelmann D, Egu DT, Steinert LS, Fuchs M, Hiermaier M, Radeva MY, Bayerbach FC, Butz E, Kotschi S, Hudemann C, Hertl M, Yeruva S, Schmidt E, Yazdi AS, Ghoreschi K, Vielmuth F, Waschke J. Apremilast prevents blistering in human epidermis and stabilizes keratinocyte adhesion in pemphigus. Nature communications. 2023 Jan 9:14(1):116. doi: 10.1038/s41467-022-35741-0. Epub 2023 Jan 9
[PubMed PMID: 36624106]
[73]
Patil S, Mustaq S, Hosmani J, Khan ZA, Yadalam PK, Ahmed ZH, Bhandi S, Awan KH. Advancement in therapeutic strategies for immune-mediated oral diseases. Disease-a-month : DM. 2023 Jan:69(1):101352. doi: 10.1016/j.disamonth.2022.101352. Epub 2022 Mar 24
[PubMed PMID: 35339251]
[74]
Bilgic A, Murrell DF. What is novel in the clinical management of pemphigus. Expert review of clinical pharmacology. 2019 Oct:12(10):973-980. doi: 10.1080/17512433.2019.1670059. Epub 2019 Sep 29
[PubMed PMID: 31550941]
[78]
Kridin K, Zelber-Sagi S, Bergman R. Pemphigus Vulgaris and Pemphigus Foliaceus: Differences in Epidemiology and Mortality. Acta dermato-venereologica. 2017 Oct 2:97(9):1095-1099. doi: 10.2340/00015555-2706. Epub
[PubMed PMID: 28536732]
[79]
Weinstein RS. Glucocorticoid-induced osteoporosis and osteonecrosis. Endocrinology and metabolism clinics of North America. 2012 Sep:41(3):595-611. doi: 10.1016/j.ecl.2012.04.004. Epub 2012 May 23
[PubMed PMID: 22877431]
[80]
Lane NE. Glucocorticoid-Induced Osteoporosis: New Insights into the Pathophysiology and Treatments. Current osteoporosis reports. 2019 Feb:17(1):1-7. doi: 10.1007/s11914-019-00498-x. Epub
[PubMed PMID: 30685820]
[82]
Namazi N, Ariaeenejad S, Azad ME, Pishgahi M. Risk of Atrial Fibrillation in Pemphigus Vulgaris. Indian dermatology online journal. 2018 Jul-Aug:9(4):275-277. doi: 10.4103/idoj.IDOJ_205_17. Epub
[PubMed PMID: 30050823]
[83]
Paracha MM, Sagheer F, Khan AQ. A clinic-epidemiological study of 148 patients of pemphigus at Lady Reading Hospital, Peshawar: a case series. JPMA. The Journal of the Pakistan Medical Association. 2023 Mar:73(3):659-662. doi: 10.47391/JPMA.6039. Epub
[PubMed PMID: 36932776]
Level 2 (mid-level) evidence
[84]
Maruyama S, Yamazaki M, Abé T, Kato Y, Kano H, Sumita Y, Tomihara K, Tanuma JI. Liquid-based cytology for differentiating two cases of pemphigus vulgaris from oral squamous cell carcinoma. Diagnostic cytopathology. 2023 May:51(5):E170-E175. doi: 10.1002/dc.25117. Epub 2023 Feb 10
[PubMed PMID: 36762831]
Level 3 (low-level) evidence
[85]
Miyachi H, Konishi T, Hashimoto Y, Matsui H, Fushimi K, Inozume T, Yasunaga H. Clinical course and outcomes of pemphigus vulgaris and foliaceus: A retrospective study using a nationwide database in Japan. The Journal of dermatology. 2023 Feb:50(2):212-221. doi: 10.1111/1346-8138.16641. Epub 2022 Nov 24
[PubMed PMID: 36424909]
Level 2 (mid-level) evidence
[86]
Pankakoski A, Kluger N, Sintonen H, Panelius J. Clinical manifestations and comorbidities of pemphigus: a retrospective case-control study in southern Finland. European journal of dermatology : EJD. 2022 Jul 1:32(4):480-486. doi: 10.1684/ejd.2022.4266. Epub
[PubMed PMID: 36301748]
Level 2 (mid-level) evidence
[87]
Karakioulaki M, Murrell DF, Kyriakou A, Patsatsi A. Investigation of comorbid autoimmune diseases in women with autoimmune bullous diseases: An interplay of autoimmunity and practical implications. International journal of women's dermatology. 2022 Oct:8(3):e053. doi: 10.1097/JW9.0000000000000053. Epub 2022 Oct 7
[PubMed PMID: 36225612]
[88]
Kadota H, Miyashita K, Fukushima S, Oryoji C, Hanada M, Yoshida S, Fujita H, Tachibana Y. Successful Management of a Severe Sacral Pressure Injury Penetrating to the Retroperitoneum. Wounds : a compendium of clinical research and practice. 2021 Mar:33(3):E24-E27
[PubMed PMID: 33914698]
[89]
Solanki VK, Nair PMK. Lifestyle medicine approach in managing pemphigus vulgaris: A case report. Explore (New York, N.Y.). 2023 Jul-Aug:19(4):617-620. doi: 10.1016/j.explore.2023.01.003. Epub 2023 Jan 7
[PubMed PMID: 36646613]
Level 3 (low-level) evidence
[90]
Czerninski R, Zadik Y, Kartin-Gabbay T, Zini A, Touger-Decker R. Dietary alterations in patients with oral vesiculoulcerative diseases. Oral surgery, oral medicine, oral pathology and oral radiology. 2014 Mar:117(3):319-23. doi: 10.1016/j.oooo.2013.08.006. Epub 2013 Oct 18
[PubMed PMID: 24144994]
[91]
Hübner S, Sarhan M, Schauer F. Use of Complementary and Alternative Medicine in Patients with Autoimmune Bullous Dermatoses: A Cohort Study Analysis of a Rare Disease Group. Complementary medicine research. 2023:30(3):221-229. doi: 10.1159/000529142. Epub 2023 Jan 16
[PubMed PMID: 36646063]