Continuing Education Activity
Partial seizures, also referred to as "focal seizures," affect a single brain area, with the temporal lobes being the most common occurrence site. They are associated with epilepsy. Despite the vast majority of partial seizures being harmless, sometimes the condition may be severe. To avoid complications, several antiepileptic medications are administered to patients with partial seizures after diagnosis. This activity reviews the evaluation and treatment of partial epilepsy and highlights the interprofessional team's role in evaluating and treating patients with this condition.
Objectives:
- Identify the pathophysiology of partial epilepsy.
- Explain the clinical exam findings associated with partial epilepsy.
- List the management options available for partial epilepsy.
Introduction
Seizure activity manifests when the brain's electrical impulses discharge abnormally, resulting in disrupting or dysregulation of normal neuronal communication. Seizures, whether isolated or recurrent, originate from the brain's cerebral cortex or hippocampus region.[1] The difference between a seizure and epilepsy depends on the number of episodes. A seizure is a single episode with a low risk of recurrence. In contrast, epilepsy is a disease in which two or more unprovoked seizures occur more than 24 hours apart, or a single seizure episode with a probability of further seizures similar to the general recurrence risk after two unprovoked seizures occurring over the next ten years, specifically, 60% or higher.[2] The diagnosis of an epileptic syndrome includes seizures associated with a common etiology, known prognosis, and accepted treatment. The predisposition of epileptic activity is determined by the underlying cause, whether known or unknown. An exception to this rule is fever in children. A child with a simple febrile seizure is typically not diagnosed with epilepsy because the seizure activity will subside when the fever resolves; however, complex febrile seizures are linked with epilepsy.
The 2017 International League Against Epilepsy classification of seizures makes a point of the onset/origin of the seizure in the brain.[3] Seizures can be categorized as partial or generalized. Seizures originating from a single location in the brain are considered to have a focal onset, known as a partial seizure. Seizure activity originating simultaneously from bilateral hemispheres is considered to have a generalized onset. Partial seizures are further divided into simple and complex. Simple partial seizures involve a small portion or a focal area of the brain. Complex partial seizures start in one area and travel to another. The main difference between a simple partial seizure and a complex partial seizure is impaired awareness and a post-ictal period in the latter, which also occurs in a generalized seizure. Thus complex partial seizures and generalized seizures may have similar presentations; however, they differ in that generalized seizures involve both hemispheres of the brain, whereas complex partial seizures travel from one focal area to another. Focal epilepsy and partial epilepsy are used interchangeably.
The international classification also divides partial seizures into two categories based on their respective etiologies: idiopathic partial seizures in which the cause is suggested to have a genetic component, and cryptogenic/symptomatic partial seizures in which the cause is acquired.[4][5] Partial seizures are further classified into simple partial seizures or seizures with auras. Aura's are sensations the patient experiences, in which the seizure activity manifests hallucinations involving visual, auditory, olfactory, tactile, or taste senses.[6] If a seizure does not follow the aura, it may be considered a focal aware seizure or simple partial.
The diagnosis of epilepsy may negatively impact psychosocial behavior and cognitive function. Therefore, patients consequently develop comorbid conditions or manifest side effects of neuro-modulating medication. Thus, there is an increasingly evident demand for concurrent interprofessional approaches to optimize the treatment of epilepsy in an effort to ensure a better quality of life.[7]
Etiology
The etiology of seizures varies widely across different age groups. Throughout the years, the influence of a genetic component in the development of partial seizures has become more prevalent.[4] Establishing the exact cause or location of epileptogenesis can be difficult, at times, only through the process of elimination. The etiology of all epilepsies is classified under one of the following categories: genetic, structural, metabolic, autoimmune, infectious, or unknown.
Mesial temporal lobe epilepsies (MTLE) are identified as the most common form of partial epilepsy. The etiology of MTLE is linked to hippocampal sclerosis. Hippocampal sclerosis is caused by the disruption of normal mRNA transcription leading to various malfunctions of healthy cells and ultimately resulting in permanent sclerotic changes.[8] As the sclerotic tissue replaces healthy functioning tissue, the patient develops resistance to anti-epileptic medications resulting in drug-resistant epilepsy.
Genetic
-
Idiopathic gene mutations with no family history of epilepsy
-
Gene mutations with a family history of epilepsy
-
Developmental disabilities
Structural
Infectious[9]
- Human immunodeficiency virus
- Subacute sclerosing panencephalitis
-
Toxoplasmosis
- Neurocysticercosis
Metabolic
Autoimmune Disorders[11]
- Sjogren syndrome
-
Sarcoidosis
-
Hashimoto encephalopathy
- Sjogren syndrome
-
Chron disease
- Behcet disease
Epidemiology
Epilepsy affects over 68 million people globally, approximately 2.2 million people in the United States. With over 150,000 new cases diagnosed yearly, epilepsy has been established as one of the most common neuronal disorders. By 20 years of age, one out of every 100 people will develop epilepsy, whereas three out of every 100 individuals will be diagnosed by age 80.
Across North America, epilepsy is more common in racial and ethnic minorities and individuals from impoverished backgrounds, with males affected at a slightly higher rate than females.[12] Partial epilepsy exhibits a bi-modal distribution affecting the young and the elderly.[12] Simple partial seizures occur in approximately 6 to 12% of patients with epilepsy.[13] Complex partial seizures occur in approximately 36% of patients with epilepsy.[14]
In children, apart from febrile convulsions, the most prevalent form of partial epilepsy is benign partial epilepsy with centrotemporal spikes (benign rolandic epilepsy) occurring between the ages of 5 and 10.[4] Glioneuronal tumors typically arise from the temporal lobe presenting with focal epilepsy more commonly in children and adolescents.[15]
Pathophysiology
The neuronal pathogenesis of epilepsy is not fully understood despite its high prevalence. Strong evidence suggests a genetic component affecting the transcription of specific principal neurons regulated by gamma-aminobutyric acid (GABA), an inhibitory neurotransmitter. Subtypes of principal and GABAergic interneurons are the most likely candidates for contributing to seizure triggering and propagation.[1]
Genetic predisposition to epilepsy has also been attributed to an upregulation of the glutamate receptor genes, an excitatory neurotransmitter. As GABA becomes deregulated, the glutamate receptor initiates an uninhibited, unsynchronized neuronal activity that generates a seizure activity. Structural lesions such as tumors, sclerotic tissue, or damaged tissue will serve as epileptogenic areas due to the disruption of normal electrical propagation.
Histopathology
Tissue excised from the epileptogenic areas may show cortical dysplasia, cortical inflammation, neoplastic/dysplastic changes, or vascular malformations. For patients diagnosed with MTLE, a post mortem autopsy will grossly show hippocampal sclerosis. On a cellular level, Hematoxylin and Eosin staining of cells excised from the sclerotic tissue will exhibit areas of dysplastic changes represented by small pink pools within the cytoplasm surrounding nucleolated nuclei.[16]
For patients presenting with tumor-associated epilepsy, the histopathological characteristics will identify the type and classification of the tumor, but these characteristics are not specific to partial epilepsy.[17] Similarly, seizures attributed to infectious and autoimmune diseases can be identified through histopathological analysis of cells acquired through tissue biopsy, a lumbar puncture, and cerebrospinal fluid analysis.
History and Physical
A detailed history and physical are vital to accurately diagnose the type of seizure, find the probable cause, and determine recurrence risk. Patients may present with:
- Tingling
- Numbness
- Jerking movements
- Muscle tightening
- Staring into space
- Rapid eye movement
- Aura sensation or hallucinations
- Smells
- Taste
- Sight
- Sounds or voices
- Tactile sensation
Clinical symptoms are determined by the anatomical areas affected, thus may facilitate the identification of the specific areas of epileptogenesis utilizing the topographical representation of the cortical homunculus.[18] Partial epilepsies show common characteristics from the areas they arise from such areas.
- Temporal - olfactory, psychomotor agitation, dysphagia
- Parietal - auditory
- Occipital - visual
- Frontal - loss of inhibition/cognition
- Postcentral gyrus - tingling, numbness
- Precentral gyrus - jerking movement, muscle tightening
Complex partial seizures may present with an aura sensation. When patients present with an aura type of partial seizures, they claim to see, taste, smell, hear, and feel a not-present sensation. Complex partial epilepsy commonly arises from the temporal lobe causing cognitive and affective symptoms such as deja vu sensation or the ability to have psychic powers; some believe they are having conversations with God.[19]
Patients can present autonomic dysfunction, motor, or sensory symptoms arising from the sensorimotor cortex in the temporal, parietal, or occipital lobes. The mouth, thumb, index finger, or great toe are extremely sensitive to electrical discharges, and often the patient describes increased sensation in these areas at the onset of seizure activity.
Evaluation
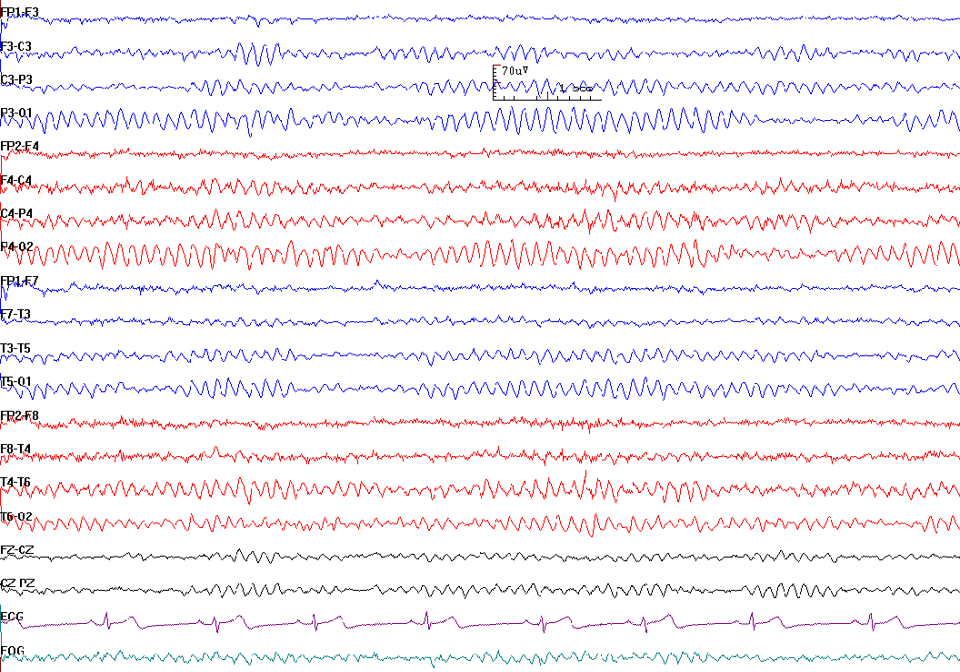
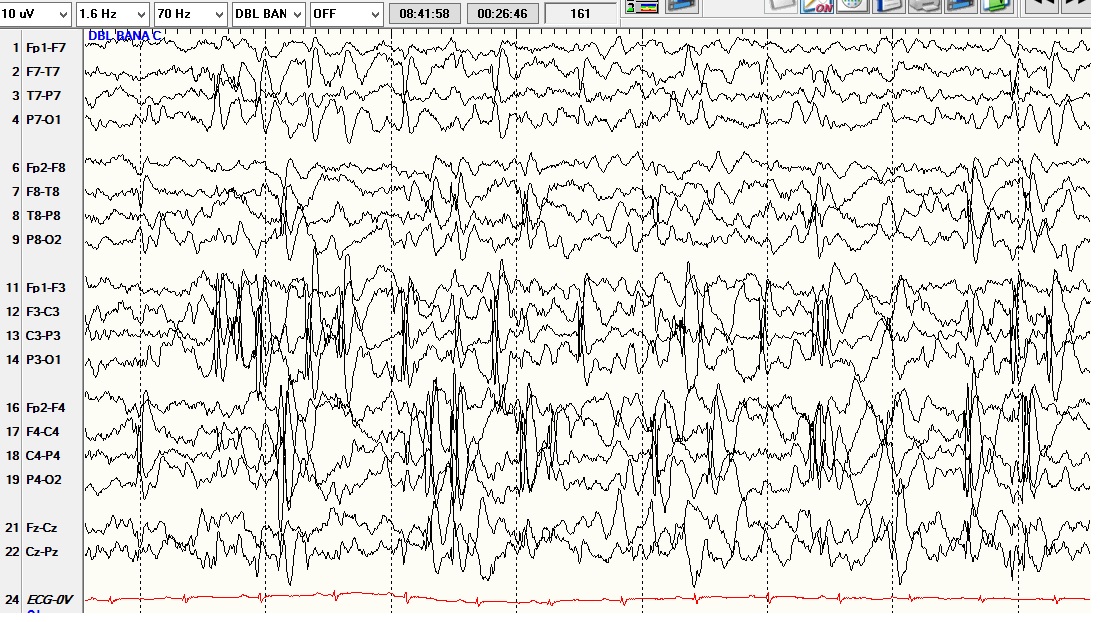
Electroencephalogram (EEG)- an EEG is useful in detecting the origin of seizure activity. In some cases, it may predict whether seizures will be controlled or uncontrolled. EEG may be normal once the seizure has been resolved. EEG can be video recorded while the patients are exposed to stressors such as sleep deprivation, light stimulation, and hyperventilation to induce seizure activity in a controlled environment. Interictal spikes seen on the EEG at a focal site are suggestive of epilepsy. A drawback to scalp EEG is that if the seizure activity is generated from deeper areas within the brain, a more invasive procedure is required. A neurosurgical procedure is necessary to place intracranial electrodes in targeted areas.
Imaging studies to identify structural causes:
- Computed tomography scan of the head with and without contrast
- Magnetic resonance imaging of the brain with fluid-attenuated inversion recovery (FLAIR) sequence
- Interictal positron emission tomography (PET)
- Ictal single-photon emission computerized tomography (SPECT)
Cardiac workup with an electrocardiogram (EKG) and Holter monitor.
Laboratory analysis includes complete blood count, comprehensive metabolic panel, drug screen, blood alcohol level, toxin screen, and thyroid panel.
Consultations with neurologists, psychologists/psychiatrists, and social workers for an interdisciplinary team approach.
Treatment / Management
First and foremost, it is essential to identify, if possible, the cause and type of seizure. Benign focal epilepsy typically self-resolves and does not require further intervention. Simple partial epilepsy can be treated with an array of medications. There is no drug of choice, and the patient should be informed that a period of trial and error may ensue. Commonly used first-line medications for partial epilepsy include carbamazepine and lamotrigine. If seizures are not controlled, a second medication can be utilized including valproate, topiramate, oxcarbazepine, or gabapentin.
Lamotrigine is the treatment of choice in the case of partial seizures associated with secondary generalization. Lamotrigine acts by blocking voltage-gated sodium channels. Lamotrigine is used in patients who do not tolerate, do not respond to, or have contraindications to valproate. Apart from partial seizures, lamotrigine is also specifically used for Lennaux Gestaut syndrome. Lamotrigine is also used in treating bipolar disorder. A severe adverse reaction associated with lamotrigine use is Stevens-Johnson syndrome, mainly when used with carbamazepine. Stevens-Johnson syndrome presents with a generalized rash, widespread bullae, and mucosal involvement. These bullae rapidly progress to denude the surface epithelium, potentially leading to severe dehydration and sepsis, among other complications. Treatment involves immediate cessation of the drug and supportive care. It can also cause toxic epidermal necrolysis.
Refractory (intractable) epilepsy has seizures that cannot be controlled with two antiepileptic medications after titrating to the highest dose and attaining therapeutic levels in the blood. In patients discontinuing medicines due to side effects, the seizures are not considered refractory.[20] Alternative treatments are considered for these patients.
- Neurostimulation treatments for intractable partial epilepsy are proven to reduce seizure activity. These treatments are well tolerated by patients and considered safe.[21] They are also beneficial for those discontinuing medication due to side effects as they reduced seizure activity.
- Surgical intervention is reserved for MTLE seizures secondary to sclerosis or seizures caused by structural lesions such as surgically excisable tumors.
Adjunct therapy with diets high in protein, such as Atkins and ketogenic diets, has reported benefits in patients experiencing refractory seizures.[22][23][24][25][26][27][28] However, close monitoring of serum concentrations of antiepileptic drugs should be done as the diet may reduce serum concentrations and cause seizure aggravation.[29]
Personalized, experimental treatment through reprogramming and reinsertion of patient-derived stem cells had shown promising results.[30]
Differential Diagnosis
Partial epilepsy has a myriad of symptomatic presentations dependent on the focal area from where the seizure activity arises. Diagnostic considerations for new-onset seizure activity should include:
- Absence seizures
- Benign childhood epilepsy
- Complex partial seizures
- Transient ischemic attacks
- Atypical migraine
- Neuromuscular disorders
- Hypoglycemia
- Alcohol withdrawal
- Drug intoxication
- Environmental toxins
- Panic attacks
- Thyroid diseases
- Malignancies
- Causing structural disruption within the brain
- Causing metabolic disturbances
- Adrenal tumors
- Renal tumors
- Gastrointestinal tumors
- Thyroid tumors
Prognosis
The prognosis for partial epilepsy in children is favorable, with over 80% reduction in seizure activity over seven years.[31] Benign childhood seizures may not require further treatment in adulthood. Epilepsy was considered a diagnosis for life; however, the Task Force deems epilepsy as resolved in patients who are seizure-free and medication-free for ten years. Terms such as "cured" and "remission" are not recommended as the risk of seizure activity does not fall to that of the general population.[2]
Complications
Uncontrolled seizures can lead to cardiac arrhythmia and possibly death.
Common side effects of medications include dizziness, nausea, vomiting, gingival hyperplasia, diarrhea. Serious and life-threatening side effects include QT prolongation on the EKG, agranulocytosis, depression, liver failure, hearing loss, pancreatitis.
Consultations
Consultation with a neurologist is the gold standard. If the underlying cause is difficult to ascertain, further consultations with cardiology, endocrine, and neurosurgery may prove beneficial.
Neuropsychiatric consultation can help diagnose the underlying cause; furthermore, this consultation serves a greater purpose as the prevalence of depression, or other concurrent psychiatric illnesses, is higher in patients suffering from chronic diseases like epilepsy.
Deterrence and Patient Education
Patients must be educated on the triggers of their seizure activity, the side effects of medication, and the disease's prognosis. Parents/guardians of minors, along with the patient, should be educated. Adult patients deemed independent should be encouraged to include and educate spouses, significant others, family members, or trusted persons to ensure their safety.
Patients should be evaluated on an individual basis for safety and risk involved with driving, swimming, recreational sports, and hazardous work environments. Uncontrolled seizures require extra safety precautions, including deterrence from driving, swimming, performing strenuous, or extreme sports activities.
Patients with epilepsy require regular follow-ups appointments and long-term monitoring to observe medication efficacy, potential side effects, and the development of comorbid conditions.
Enhancing Healthcare Team Outcomes
Partial epilepsy strongly affects a patient's lifestyle, secondary to the disruption of normal neurological processes. Patient-centered care requires an interdisciplinary, interprofessional team coordinating an evidence-based and holistic approach to optimizing patient outcomes and bettering their quality of life. The patient should have adequate follow-up care to assess for side effects of medication, medication adherence, clinical symptoms, accessibility to specialists, and comorbid conditions. Thus, an interdisciplinary team to monitor and communicate between the primary care physician, neurologist, psychiatrist, pharmacist, and social worker is mandatory.
The Program to Encourage Active Rewarding Lives for Seniors (PEARLS) enrolled older adults into a home-based program that integrated the patient with community outreach to detect and manage minor depression or dysthymia.[32] There is an increased risk of depression and suicide, regardless of age, linked to epileptic patients. In a randomized clinical trial that enrolled adult epileptic participants using the PEARLS program, a significant reduction in depression was obtained, with effects lasting more than one year after the end of the home visits.[33][34] [Level 1] The PEARLS program had been utilized by the Epilepsy Foundation to decrease depressive symptoms and improve the quality of life of adult patients with epilepsy using brief behavioral techniques during 6 to 8 home sessions.