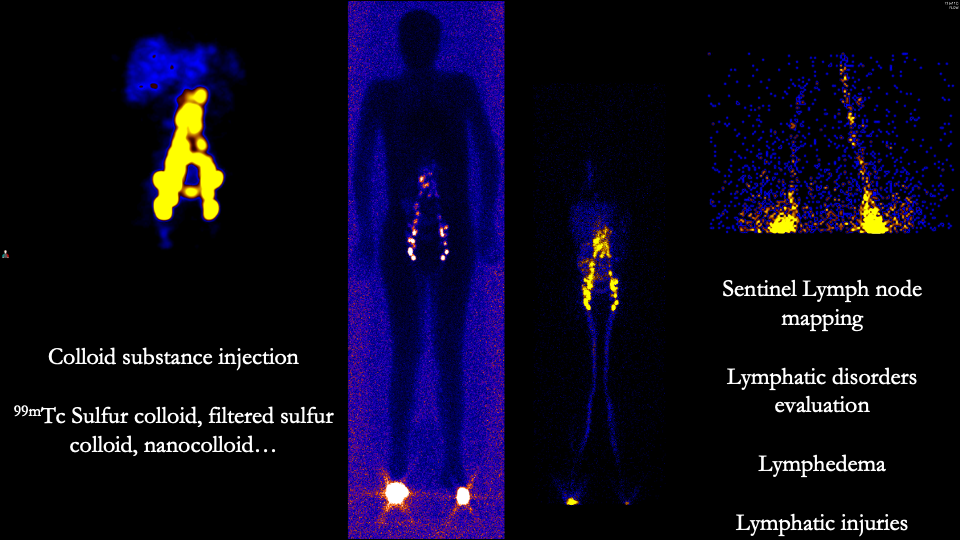
[1]
Suy R, Thomis S, Fourneau I. The discovery of the lymphatic system in the seventeenth century. Part II: the discovery of Chyle vessels. Acta chirurgica Belgica. 2016 Oct:116(5):329-335. doi: 10.1080/00015458.2016.1195587. Epub 2016 Aug 26
[PubMed PMID: 27563735]
[2]
Suami H, Pan WR, Taylor GI. Historical review of breast lymphatic studies. Clinical anatomy (New York, N.Y.). 2009 Jul:22(5):531-6. doi: 10.1002/ca.20812. Epub
[PubMed PMID: 19484798]
[3]
Tanis PJ, Nieweg OE, Valdés Olmos RA, Th Rutgers EJ, Kroon BB. History of sentinel node and validation of the technique. Breast cancer research : BCR. 2001:3(2):109-12
[PubMed PMID: 11250756]
Level 1 (high-level) evidence
[4]
Kazem I, Antoniades J, Brady LW, Faust DS, Croll MN, Lightfoot D. Clinical evaluation of lymph node scanning utilizing colloidal gold 198. Radiology. 1968 May:90(5):905-11
[PubMed PMID: 5643593]
[5]
Moore JE Jr, Bertram CD. Lymphatic System Flows. Annual review of fluid mechanics. 2018 Jan:50():459-482. doi: 10.1146/annurev-fluid-122316-045259. Epub
[PubMed PMID: 29713107]
[6]
Cirocchi R, Metaj G, Cicoletti M, Arcangeli F, De Sol A, Poli G, Bruzzone P, Gioia S, Anagnostou C, Loreti F, Francesconi S, Ricci L, Laurenti ME, Capotorti A, Artico M, D'Andrea V, Henry BM, Fedeli P, Carlini L. Analysis of the Different Lymphatic Drainage Patterns during Sentinel Lymph Node Biopsy for Skin Melanoma. Journal of clinical medicine. 2021 Nov 26:10(23):. doi: 10.3390/jcm10235544. Epub 2021 Nov 26
[PubMed PMID: 34884243]
[7]
Reynolds HM, Walker CG, Dunbar PR, O'Sullivan MJ, Uren RF, Thompson JF, Smith NP. Functional anatomy of the lymphatics draining the skin: a detailed statistical analysis. Journal of anatomy. 2010 Mar:216(3):344-55. doi: 10.1111/j.1469-7580.2009.01183.x. Epub 2010 Jan 7
[PubMed PMID: 20070428]
[8]
Suami H, Koelmeyer L, Mackie H, Boyages J. Patterns of lymphatic drainage after axillary node dissection impact arm lymphoedema severity: A review of animal and clinical imaging studies. Surgical oncology. 2018 Dec:27(4):743-750. doi: 10.1016/j.suronc.2018.10.006. Epub 2018 Oct 12
[PubMed PMID: 30449502]
Level 3 (low-level) evidence
[9]
Zhou Y, Hou J, Meng N, Daniel S, Chen J, Xu L. Case Report: Intercostal Lymph Node Metastasis: A Case Report and Review of the Literature. Frontiers in oncology. 2021:11():638948. doi: 10.3389/fonc.2021.638948. Epub 2021 Mar 4
[PubMed PMID: 33747962]
Level 3 (low-level) evidence
[11]
Burke TW, Levenback C, Tornos C, Morris M, Wharton JT, Gershenson DM. Intraabdominal lymphatic mapping to direct selective pelvic and paraaortic lymphadenectomy in women with high-risk endometrial cancer: results of a pilot study. Gynecologic oncology. 1996 Aug:62(2):169-73
[PubMed PMID: 8751545]
Level 3 (low-level) evidence
[12]
Herbert GS, Beshlian KM. The triangular intermuscular space as a site of lymph node metastasis in melanoma of the back. Annals of plastic surgery. 2010 Jan:64(1):52-4. doi: 10.1097/SAP.0b013e31819b6c87. Epub
[PubMed PMID: 20023455]
[13]
Kalawat TC, Chittoria RK, Reddy PK, Suneetha B, Narayan R, Ravi P. Role of lymphoscintigraphy in diagnosis and management of patients with leg swelling of unclear etiology. Indian journal of nuclear medicine : IJNM : the official journal of the Society of Nuclear Medicine, India. 2012 Oct:27(4):226-30. doi: 10.4103/0972-3919.115392. Epub
[PubMed PMID: 24019651]
[14]
Zhang L, Zhang X, Wen Z, Tong G, Hao K, Qiu Y, Kang L. Lymphoscintigraphy findings in patients with chylothorax: influence of biochemical parameters. EJNMMI research. 2023 Aug 3:13(1):72. doi: 10.1186/s13550-023-01014-0. Epub 2023 Aug 3
[PubMed PMID: 37535169]
Level 2 (mid-level) evidence
[15]
Slomovitz BM, Coleman RL, Oonk MH, van der Zee A, Levenback C. Update on sentinel lymph node biopsy for early-stage vulvar cancer. Gynecologic oncology. 2015 Aug:138(2):472-7. doi: 10.1016/j.ygyno.2015.05.017. Epub 2015 May 27
[PubMed PMID: 26022527]
[16]
Lécuru F, Mathevet P, Querleu D, Leblanc E, Morice P, Daraï E, Marret H, Magaud L, Gillaizeau F, Chatellier G, Dargent D. Bilateral negative sentinel nodes accurately predict absence of lymph node metastasis in early cervical cancer: results of the SENTICOL study. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011 May 1:29(13):1686-91. doi: 10.1200/JCO.2010.32.0432. Epub 2011 Mar 28
[PubMed PMID: 21444878]
[17]
Giammarile F, Alazraki N, Aarsvold JN, Audisio RA, Glass E, Grant SF, Kunikowska J, Leidenius M, Moncayo VM, Uren RF, Oyen WJ, Valdés Olmos RA, Vidal Sicart S. The EANM and SNMMI practice guideline for lymphoscintigraphy and sentinel node localization in breast cancer. European journal of nuclear medicine and molecular imaging. 2013 Dec:40(12):1932-47. doi: 10.1007/s00259-013-2544-2. Epub 2013 Oct 2
[PubMed PMID: 24085499]
Level 1 (high-level) evidence
[18]
Chen SL, Iddings DM, Scheri RP, Bilchik AJ. Lymphatic mapping and sentinel node analysis: current concepts and applications. CA: a cancer journal for clinicians. 2006 Sep-Oct:56(5):292-309; quiz 316-7
[PubMed PMID: 17005598]
[19]
Fisher B, Anderson S. Conservative surgery for the management of invasive and noninvasive carcinoma of the breast: NSABP trials. National Surgical Adjuvant Breast and Bowel Project. World journal of surgery. 1994 Jan-Feb:18(1):63-9
[PubMed PMID: 8197778]
[20]
Giuliano AE, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Hunt KK, Morrow M, Ballman K. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Annals of surgery. 2010 Sep:252(3):426-32; discussion 432-3. doi: 10.1097/SLA.0b013e3181f08f32. Epub
[PubMed PMID: 20739842]
Level 1 (high-level) evidence
[21]
Giuliano AE, Ballman K, McCall L, Beitsch P, Whitworth PW, Blumencranz P, Leitch AM, Saha S, Morrow M, Hunt KK. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Annals of surgery. 2016 Sep:264(3):413-20. doi: 10.1097/SLA.0000000000001863. Epub
[PubMed PMID: 27513155]
Level 1 (high-level) evidence
[22]
Faries MB, Cochran AJ, Elashoff RM, Thompson JF. Multicenter Selective Lymphadenectomy Trial-I confirms the central role of sentinel node biopsy in contemporary melanoma management: response to 'No survival benefit for patients with melanoma undergoing sentinel lymph node biopsy: critical appraisal of the Multicenter Selective Lymphadenectomy Trial-I final report'. The British journal of dermatology. 2015 Mar:172(3):571-3. doi: 10.1111/bjd.13676. Epub
[PubMed PMID: 25776247]
[23]
Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, Jahkola T, Bowles TL, Testori A, Beitsch PD, Hoekstra HJ, Moncrieff M, Ingvar C, Wouters MWJM, Sabel MS, Levine EA, Agnese D, Henderson M, Dummer R, Rossi CR, Neves RI, Trocha SD, Wright F, Byrd DR, Matter M, Hsueh E, MacKenzie-Ross A, Johnson DB, Terheyden P, Berger AC, Huston TL, Wayne JD, Smithers BM, Neuman HB, Schneebaum S, Gershenwald JE, Ariyan CE, Desai DC, Jacobs L, McMasters KM, Gesierich A, Hersey P, Bines SD, Kane JM, Barth RJ, McKinnon G, Farma JM, Schultz E, Vidal-Sicart S, Hoefer RA, Lewis JM, Scheri R, Kelley MC, Nieweg OE, Noyes RD, Hoon DSB, Wang HJ, Elashoff DA, Elashoff RM. Completion Dissection or Observation for Sentinel-Node Metastasis in Melanoma. The New England journal of medicine. 2017 Jun 8:376(23):2211-2222. doi: 10.1056/NEJMoa1613210. Epub
[PubMed PMID: 28591523]