Introduction
Many glial cells provide support for an essential nervous system function. In addition to providing support for neurons, glial cells aid in the maintenance of homeostasis, and form myelin. As a whole, glial cells are the most abundant cells in the central nervous system. The most notable glial cells include oligodendrocytes, Schwann cells, astrocytes, microglia, and ependymal cells. Most glial cells are capable of mitotic division.
Structure
Oligodendrocytes have small nuclei that are surrounded by rings of cytoplasm. They have long cytoplasmic projections that extend from the soma. The cytoplasm is notable for an abundance of polyribosomes and granular endoplasmic reticulum. Interfascicular oligodendrocytes are those between myelinated axons, and satellite oligodendrocytes are prevalent in gray matter and are those adjacent to cell bodies.
Schwann cells are the equivalent of oligodendrocytes in the peripheral nervous system. Unlike oligodendrocytes, each Schwann cell is only capable of myelinating a single axon. Schwann cells have elongated nuclei and exhibit an elongated, tubular shape. Schwann cells wrap closely around axons as the axons pass through the Schwann cell cytoplasm.
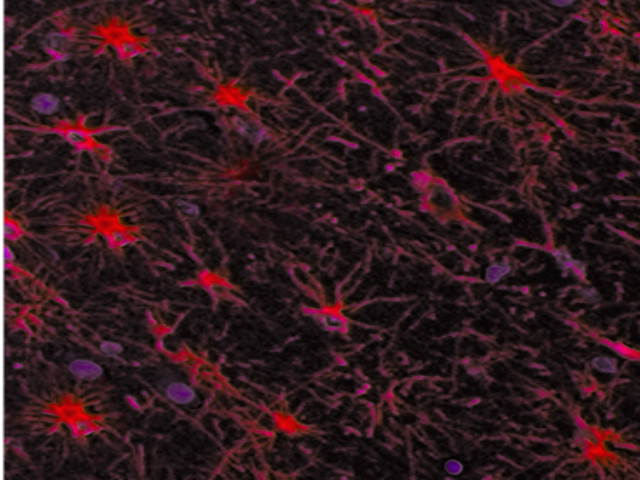
Astrocytes are star-shaped glial cells that have numerous processes extending from them. Their foot processes are an important component of the blood-brain barrier. The branching of astrocytic processes enables their contact with many neuronal soma, dendrites, and axons simultaneously. Protoplasmic astrocytes are found primarily in the gray matter and are involved in synaptic processes and neurotransmitter transport. The branches of protoplasmic astrocytes largely associate with the terminal parts of axons, as well as synapses and dendrites. Protoplasmic astrocytes exhibit a profusion of potassium channels that are involved in the diffusion of electrical signal spread to neighboring neurons. Fibrous astrocytes are more prevalent in the white matter. They are the type of astrocytes that contain large amounts of cytoplasmic GFAP in non-pathological conditions.
Microglia are small, relatively sparse cells, and are difficult to observe in microscopy. They exhibit elongated nuclei with little cytoplasm. Microglia are of monocyte origin and fill a role as immune effector cells in the central nervous system (CNS).
Ependymal cells are epithelial cells that line the ventricular system. They may exhibit either simple cuboidal or simple columnar morphology (the distinction between the two is quite subjective). Choroidal epithelial cells are involved in the regulation of cerebrospinal fluid (CSF) contents. Ependymocytes are relatively abundant and are involved in the connection between the CSF and nervous tissue. Tanycytes are most prevalent along the floor of the third ventricle.[1][2][3]
Function
Schwann cells and oligodendrocytes are the myelin-forming cells of the nervous system. For the most part, Schwann cells are present in the periphery, and oligodendrocytes are in the central nervous system; however, Schwann cells can invade into the CNS when the glial-limiting membrane becomes disrupted. Myelin is composed of layered phospholipid membranes and serves to support and insulate axons, allowing for faster impulse transduction. Saltatory conduction occurs as the impulses jump across sodium ion-rich nodes of Ranvier. One oligodendrocyte myelinates multiple axons. Oligodendrocytes are in general terminally differentiated due to exposure to transcription factor Sox10, and incapable of replication upon injury.[4]
Astrocytes are the most abundant cells in the brain. They communicate through gap junctions and are involved in the release and uptake of chemicals at synapses. Astrocytes have glial fibrillary acidic protein (GFAP) containing processes. They are involved in gliosis in response to injury. Astrocytes provide substrates for the production of ATP for neurons and are involved in potassium metabolism and water uptake for the maintenance of homeostasis. The foot processes of astrocytes that surround capillaries or extend to subpial or subependymal zones also play an important role in the blood-brain-barrier structure and function.[5]
Microglia are the mesoderm-derived, resident macrophages of the CNS. As such, they phagocytose and remove foreign or damaged material, cells, or organisms. They first proliferate, then extend the length of their nuclei, then either form aggregates around tissue necrosis or form aggregates around the cell bodies of dying neurons. Upon activation, astrocytes can also release reactive oxygen species (ROS) such as COX-2, CB2, and P2X7. The belief is that these ROS may contribute to neurodegenerative conditions such as amyotrophic lateral sclerosis and multiple sclerosis.[6]
Ependymal cells line the ventricles and fluid-filled compartments of the CNS and provide a semi-permeable layer. Ependymal cells are involved in cerebral spinal fluid (CSF) secretion. The ependyma detoxifies a variety of substances in the CSF in its role as a protective barrier between the brain and the CSF. The ependyma is also a source of NSCs which are capable of differentiating into neurons and glial cells. In cases of inflammation or ventricular dilation, ependymal cells can combine with subependymal astrocytes in the formation of ependymal granulations; however, this response to insult does not appear to be significant in most cases.[7][8]
Histochemistry and Cytochemistry
Astrocytes are characterized by the intermediate filament glial fibrillary acidic protein (GFAP). GFAP is present in abundance in the astrocytic cytoplasm. Other markers specific to astrocytes include EAAT1/GLAST and EAAT2/GLT-1. The cytoplasm of oligodendrocytes does not contain GFAP, but markers commonly used for the characterization of oligodendrocytes include oligodendrocyte specific protein (OSP), and myelin oligodendrocyte glycoprotein (MOG). Schwann cells express the intermediate filament vimentin. Both Schwann cells and oligodendrocytes express myelin basic protein (MBP). The intermediate filament vimentin is present in microglia, and nestin-positive microglia may be present.[1][2][3]
Clinical Significance
Astrocytes can form scars following injury as they hypertrophy or divide to fill spaces resulting from cavitation of neural tissue. Astrocytes also assist microglia in the regulation of the immune response to pathological insult in the nervous system by secreting interleukin-1 (IL-1) and prostaglandins, among other factors. Astrogliosis can cause the release of inhibitory molecules that prevent nervous system recovery. However, its impairment correlates with defects in the blood-brain barrier. While reactive astrocytes are the predominant cell type involved in glial scar formation, microglia, and endothelial cells and fibroblasts also play a role.
In demyelinating diseases such as multiple sclerosis and Guillain-Barré Syndrome, oligodendrocytes and/or Schwann cells degenerate in clusters. The myelin in such conditions is replaced by plaques that inhibit the transmission of action potentials along the axons. Remyelination of axons is possible in certain situations.[9][10]