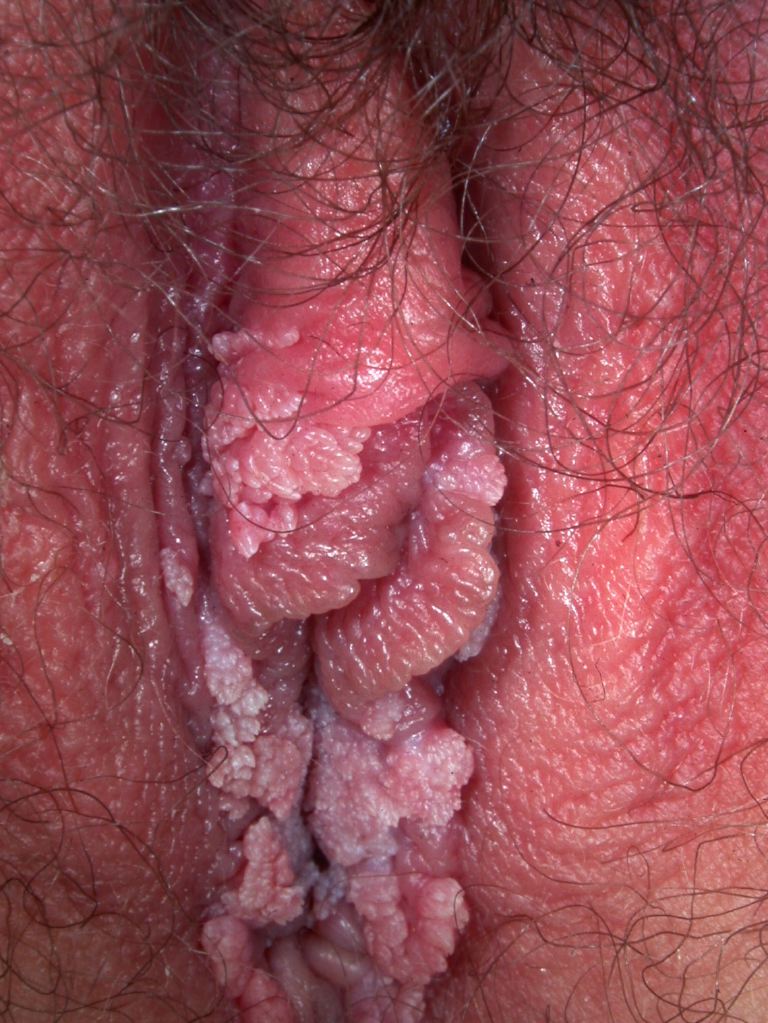
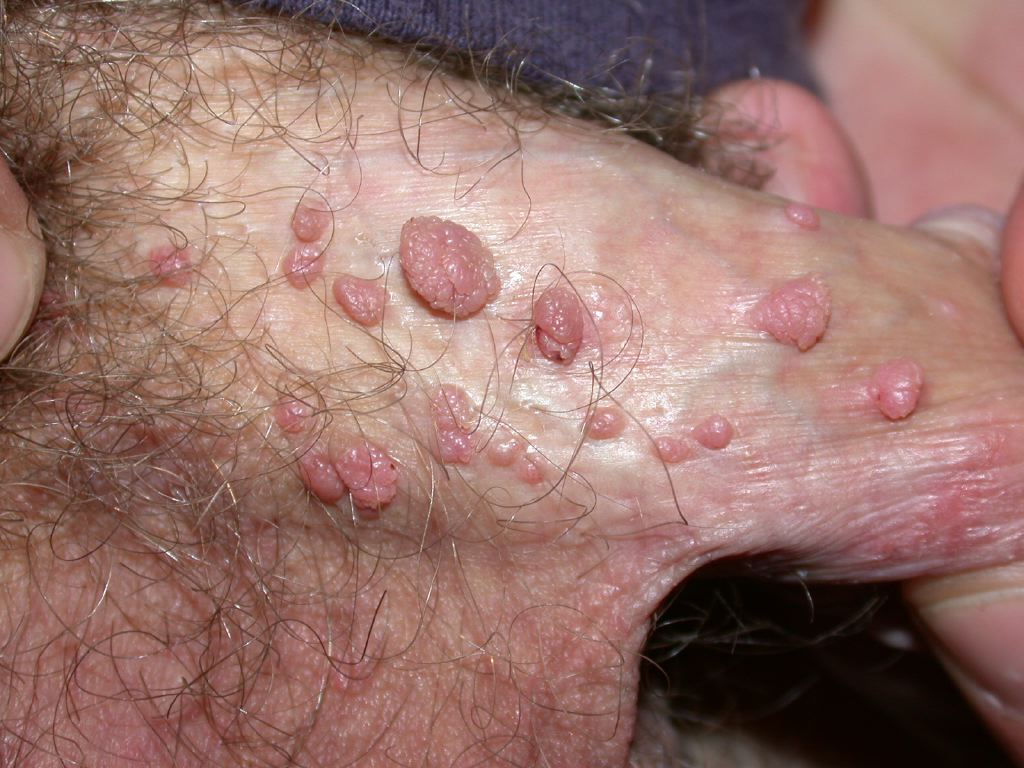
[1]
Sendagorta-Cudós E,Burgos-Cibrián J,Rodríguez-Iglesias M, Genital infections due to the human papillomavirus. Enfermedades infecciosas y microbiologia clinica (English ed.). 2019 May
[PubMed PMID: 30853139]
[2]
Ozaydin-Yavuz G,Bilgili SG,Guducuoglu H,Yavuz IH,Elibuyuk-Aksac S,Karadag AS, Determinants of high-risk human papillomavirus infection in anogenital warts. Postepy dermatologii i alergologii. 2019 Feb
[PubMed PMID: 30858783]
[3]
Lisboa C,Santo I,Azevedo J,Azevedo L,Pista A,Dias C,Cunha MJ, High Prevalence of Human Papillomavirus on Anal and Oral Samples from Men and Women with External Anogenital Warts: The HERCOLES Study. Acta dermato-venereologica. 2019 May 1
[PubMed PMID: 30723872]
[4]
Stratton KL,Culkin DJ, A Contemporary Review of HPV and Penile Cancer. Oncology (Williston Park, N.Y.). 2016 Mar
[PubMed PMID: 26984219]
[5]
Sichero L,Giuliano AR,Villa LL, Human Papillomavirus and Genital Disease in Men: What We Have Learned from the HIM Study. Acta cytologica. 2019
[PubMed PMID: 30799416]
[6]
Leung AK,Barankin B,Leong KF,Hon KL, Penile warts: an update on their evaluation and management. Drugs in context. 2018
[PubMed PMID: 30622585]
[7]
Zayko MO,Velilla RE,Shurbaji MS, Condyloma Acuminata Presenting as Isolated Papillary Lesions in the Prostatic Urethra. The American journal of case reports. 2018 Dec 22
[PubMed PMID: 30578409]
Level 3 (low-level) evidence
[8]
Iskander M,Patrick N,Mistry R, Intra urethral human papillomavirus related warts following urinary tract instrumentation. Urology journal. 2014 May 6
[PubMed PMID: 24807766]
[9]
Beutner KR,Reitano MV,Richwald GA,Wiley DJ, External genital warts: report of the American Medical Association Consensus Conference. AMA Expert Panel on External Genital Warts. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 1998 Oct;
[PubMed PMID: 9798036]
Level 3 (low-level) evidence
[10]
Wiley DJ,Douglas J,Beutner K,Cox T,Fife K,Moscicki AB,Fukumoto L, External genital warts: diagnosis, treatment, and prevention. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2002 Oct 15;
[PubMed PMID: 12353208]
[11]
Workowski KA,Bolan GA,Centers for Disease Control and Prevention., Sexually transmitted diseases treatment guidelines, 2015. MMWR. Recommendations and reports : Morbidity and mortality weekly report. Recommendations and reports. 2015 Jun 5;
[PubMed PMID: 26042815]
[12]
Murray ML,Meadows J,Doré CJ,Copas AJ,Haddow LJ,Lacey C,Jit M,Soldan K,Bennett K,Tetlow M,Nathan M,Gilson R, Human papillomavirus infection: protocol for a randomised controlled trial of imiquimod cream (5%) versus podophyllotoxin cream (0.15%), in combination with quadrivalent human papillomavirus or control vaccination in the treatment and prevention of recurrence of anogenital warts (HIPvac trial). BMC medical research methodology. 2018 Nov 6
[PubMed PMID: 30400777]
Level 1 (high-level) evidence
[13]
Brown TJ,Yen-Moore A,Tyring SK, An overview of sexually transmitted diseases. Part II. Journal of the American Academy of Dermatology. 1999 Nov;
[PubMed PMID: 10534627]
Level 3 (low-level) evidence
[14]
Tyring S,Edwards L,Cherry LK,Ramsdell WM,Kotner S,Greenberg MD,Vance JC,Barnum G,Dromgoole SH,Killey FP,Toter T, Safety and efficacy of 0.5% podofilox gel in the treatment of anogenital warts. Archives of dermatology. 1998 Jan;
[PubMed PMID: 9449907]
[15]
Petersen CS,Weismann K, Quercetin and kaempherol: an argument against the use of podophyllin? Genitourinary medicine. 1995 Apr;
[PubMed PMID: 7744421]
[16]
Severson J,Evans TY,Lee P,Chan T,Arany I,Tyring SK, Human papillomavirus infections: epidemiology, pathogenesis, and therapy. Journal of cutaneous medicine and surgery. 2001 Jan-Feb;
[PubMed PMID: 11281434]
[17]
Gotovtseva EP,Kapadia AS,Smolensky MH,Lairson DR, Optimal frequency of imiquimod (aldara) 5% cream for the treatment of external genital warts in immunocompetent adults: a meta-analysis. Sexually transmitted diseases. 2008 Apr;
[PubMed PMID: 18360317]
Level 1 (high-level) evidence
[18]
Czelusta AJ,Evans T,Arany I,Tyring SK, A guide to immunotherapy of genital warts: focus on interferon and imiquimod. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 1999 May;
[PubMed PMID: 18031142]
[19]
Berman B,Wolf J, The role of imiquimod 3.75% cream in the treatment of external genital warts. Skin therapy letter. 2012 Apr;
[PubMed PMID: 22491804]
Level 3 (low-level) evidence
[20]
Rosen T,Nelson A,Ault K, Imiquimod cream 2.5% and 3.75% applied once daily to treat external genital warts in men. Cutis. 2015 Oct;
[PubMed PMID: 26682290]
[21]
Tatti S,Swinehart JM,Thielert C,Tawfik H,Mescheder A,Beutner KR, Sinecatechins, a defined green tea extract, in the treatment of external anogenital warts: a randomized controlled trial. Obstetrics and gynecology. 2008 Jun;
[PubMed PMID: 18515521]
Level 1 (high-level) evidence
[22]
Schöfer H,Tatti S,Lynde CW,Skerlev M,Hercogová J,Rotaru M,Ballesteros J,Calzavara-Pinton P, Sinecatechins and imiquimod as proactive sequential therapy of external genital and perianal warts in adults. International journal of STD & AIDS. 2017 Dec
[PubMed PMID: 28566057]
[23]
Jha AK,Sonthalia S,Ganguly S, Oral isotretinoin as an adjunctive treatment for recurrent genital warts. Journal of the American Academy of Dermatology. 2018 Feb
[PubMed PMID: 29332721]
[24]
Nickle SB,Peterson N,Peterson M, Updated Physician's Guide to the Off-label Uses of Oral Isotretinoin. The Journal of clinical and aesthetic dermatology. 2014 Apr
[PubMed PMID: 24765227]
[25]
Yew YW,Pan JY, Complete remission of recalcitrant genital warts with a combination approach of surgical debulking and oral isotretinoin in a patient with systemic lupus erythematosus. Dermatologic therapy. 2014 Mar-Apr
[PubMed PMID: 24703263]
[26]
Oren-Shabtai M,Snast I,Lapidoth M,Sherman S,Noyman Y,Mimouni D,Hodak E,Levi A, Topical and Systemic Retinoids for the Treatment of Genital Warts: A Systematic Review and Meta-Analysis. Dermatology (Basel, Switzerland). 2021;
[PubMed PMID: 33279886]
Level 1 (high-level) evidence
[27]
Paredes-Bhushan V,Rezaee ME,Chavez DR, Isotretinoin induced urethritis: A case report
[PubMed PMID: 31908966]
Level 3 (low-level) evidence
[28]
Godley MJ,Bradbeer CS,Gellan M,Thin RN, Cryotherapy compared with trichloroacetic acid in treating genital warts. Genitourinary medicine. 1987 Dec;
[PubMed PMID: 3323028]
[29]
Fichman Y,Levi A,Hodak E,Halachmi S,Mazor S,Wolf D,Caplan O,Lapidoth M, Efficacy of pulsed dye laser treatment for common warts is not influenced by the causative HPV type: a prospective study. Lasers in medical science. 2018 May
[PubMed PMID: 29218494]
[30]
Iranmanesh B,Khalili M,Zartab H,Amiri R,Aflatoonian M, Laser therapy in cutaneous and genital warts: A review article. Dermatologic therapy. 2021 Jan;
[PubMed PMID: 33314577]
[31]
Xie F,Yu HS,Wang R,Wang D,Li YM,Wen HY,Du JB,Ba W,Meng XF,Yang J,Lin BW,Li HJ,Li CX,Zhang LG,Fang XD,Zhao H, Photodynamic Therapy for Genital Warts Causes Activation of Local Immunity. Journal of cutaneous medicine and surgery. 2019 Jul/Aug;
[PubMed PMID: 31010295]
[32]
Tu P,Zhang H,Zheng H,Gu H,Xu J,Tao J,Wang H,Zhu X,Wang X, 5-Aminolevulinic photodynamic therapy versus carbon dioxide laser therapy for small genital warts: A multicenter, randomized, open-label trial. Journal of the American Academy of Dermatology. 2021 Mar;
[PubMed PMID: 31374308]
Level 1 (high-level) evidence
[33]
Kechichian E,Helou E,Sarkis J,Hayek C,Labaki C,Nemr E,Tomb R, The place of 5-aminolaevulinic acid-photodynamic therapy in the treatment landscape of urethral warts: A systematic review. Photodiagnosis and photodynamic therapy. 2021 Mar;
[PubMed PMID: 33529745]
Level 1 (high-level) evidence
[34]
Zhang H,Shi L,Zhang Y,Wang P,Zhang G,Cao Y,Zhou Z,Wang X, Modified photodynamic therapy to minimize pain in the treatment of condylomata acuminata: A prospective, randomized, self-controlled study. Photodiagnosis and photodynamic therapy. 2020 Dec;
[PubMed PMID: 32634656]
Level 1 (high-level) evidence
[35]
Czelusta A,Yen-Moore A,Van der Straten M,Carrasco D,Tyring SK, An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV-infected patients. Journal of the American Academy of Dermatology. 2000 Sep
[PubMed PMID: 10954653]
Level 3 (low-level) evidence
[36]
Nebesio CL,Mirowski GW,Chuang TY, Human papillomavirus: clinical significance and malignant potential. International journal of dermatology. 2001 Jun
[PubMed PMID: 11589741]
[37]
Orlando G,Fasolo MM,Beretta R,Merli S,Cargnel A, Combined surgery and cidofovir is an effective treatment for genital warts in HIV-infected patients. AIDS (London, England). 2002 Feb 15
[PubMed PMID: 11834957]
[38]
Kaliterna V,Barisic Z, Genital human papillomavirus infections. Frontiers in bioscience (Landmark edition). 2018 Mar 1
[PubMed PMID: 29293452]
[39]
Cusini M,Micali G,Lacarrubba F,Puviani M,Barcella A,Milani M, Efficacy and tolerability of nitric-zinc complex in the treatment of external genital warts and
[PubMed PMID: 26513041]
[40]
Pontini P,Mastorino L,Gaspari V,Granger C,Ramoni S,Delmonte S,Evangelista V,Cusini M, A Multicentre, Randomised Clinical Trial to Compare a Topical Nitrizinc{sup}®{/sup} Complex Solution Versus Cryotherapy for the Treatment of Anogenital Warts. Dermatology and therapy. 2020 Oct;
[PubMed PMID: 32734366]
Level 1 (high-level) evidence
[41]
Muffarrej D,Khattab E,Najjar R, Successful treatment of genital warts with cidofovir cream in a pediatric patient with Fanconi anemia. Journal of oncology pharmacy practice : official publication of the International Society of Oncology Pharmacy Practitioners. 2020 Jul
[PubMed PMID: 31718429]
[42]
Hengge UR,Tietze G, Successful treatment of recalcitrant condyloma with topical cidofovir. Sexually transmitted infections. 2000 Apr
[PubMed PMID: 10858720]
[43]
Schürmann D,Bergmann F,Temmesfeld-Wollbrück B,Grobusch MP,Suttorp N, Topical cidofovir is effective in treating extensive penile condylomata acuminata. AIDS (London, England). 2000 May 26
[PubMed PMID: 10853999]
[44]
Blaizot R,Dutkiewicz AS,Guillet S,Pham-Ledard A,Beylot-Barry M, Intravenous cidofovir for diffuse genital warts in the setting of multifactorial immunosuppression. Journal of the European Academy of Dermatology and Venereology : JEADV. 2017 Mar;
[PubMed PMID: 27527116]
[45]
Psomiadou V,Iavazzo C,Douligeris A,Fotiou A,Prodromidou A,Blontzos N,Karavioti E,Vorgias G, An Alternative Treatment for Vaginal Cuff Wart: a Case Report. Acta medica (Hradec Kralove). 2020;
[PubMed PMID: 32422116]
Level 3 (low-level) evidence