Continuing Education Activity
A fibula free flap is a procedure commonly used in reconstructive surgery to replace missing bone and soft tissue, often in mandibular or maxillofacial reconstruction following trauma, cancer resection, or congenital deformities. The procedure involves harvesting a segment of the fibula bone along with its associated blood vessels and soft tissue, which is then transplanted to the recipient site to restore function and aesthetics. The fibula's length, shape, and dual blood supply make it an ideal choice for reconstructions, offering stability and potential for osseointegration with dental implants. This procedure requires a comprehensive understanding of vascular anatomy, microsurgical techniques, and postoperative management to ensure successful outcomes and minimize complications.
Clinicians participating in this course on fibula free flap reconstruction can expect to gain comprehensive knowledge and skills essential for proficiently performing this intricate surgical procedure. The course aims to provide clinicians with the practical skills and knowledge necessary to execute the procedure, including patient selection, preoperative planning, flap harvest, microvascular anastomosis, and postoperative monitoring. Additionally, participants will learn strategies for managing potential complications and optimizing patient outcomes, ultimately improving their ability to deliver high-quality care to patients requiring complex reconstructive surgery.
Objectives:
Identify appropriate candidates for fibula free flap reconstruction based on thorough clinical evaluation, including assessment of the extent of bone and soft tissue loss, vascular status, comorbidities, and patient preferences.
Implement meticulous preoperative planning, including detailed imaging studies, vascular mapping, and coordination with the surgical team, to ensure the successful execution of fibula free flap reconstruction.
Select appropriate postoperative monitoring protocols to assess flap viability, manage complications, and facilitate timely intervention when necessary.
Coordinate comprehensive postoperative care, including wound management, physical therapy, and long-term follow-up, to promote successful recovery and patient satisfaction.
Introduction
Bony defects commonly result from trauma, tumors, infections, or congenital anomalies, potentially leading to diminished quality of life and, in severe cases, limb amputation. Although newer methods like bridging endoprostheses and distraction osteogenesis have been developed, nonvascularized bone grafts continue to manage nonunions and short bone defects to promote healing. Bone grafting has been used in reconstructive surgery for over a century, and recent advances in microsurgical techniques have made vascularized free fibula flap transfer a viable option for reconstructing long bony defects.[1][2]
In 1975, Taylor et al pioneered the harvest and transfer of the first free fibula using a posterior approach. Gilbert subsequently refined the technique with a lateral approach, further enhanced by Chuang et al, who popularized the osteomyocutaneous fibular flap.[3][4] In 1994, Hidalgo expanded indications for fibular free tissue transfer by introducing osteotomy techniques (closing ostectomies) to shape the fibula, enabling it to mimic the mandible's contour. This innovation solidified the fibula as the cornerstone for head and neck reconstruction, which is widely utilized today.[5]
Various methods exist for employing fibular bone for reconstruction, including cancellous and cortical autografts, bone allografts, endoprosthetic replacement, vascularized bone grafts, fibular osteocutaneous flaps, and fibular osteomuscular flaps.[6][7][8] Among these, free vascularized fibular grafting stands out for its ability to offer immediate mechanical support and potential for growth or hypertrophy based on the patient's growth and activity levels.[9] While vascularized fibular grafting may not be suitable for all cases requiring bony reconstruction, it is frequently utilized for defects larger than 6 cm, failed nonvascularized bone grafting, infected nonunion with a bony defect, or following tumor resection, especially when postoperative radiation therapy is anticipated.
Anatomy and Physiology
Bony Anatomy
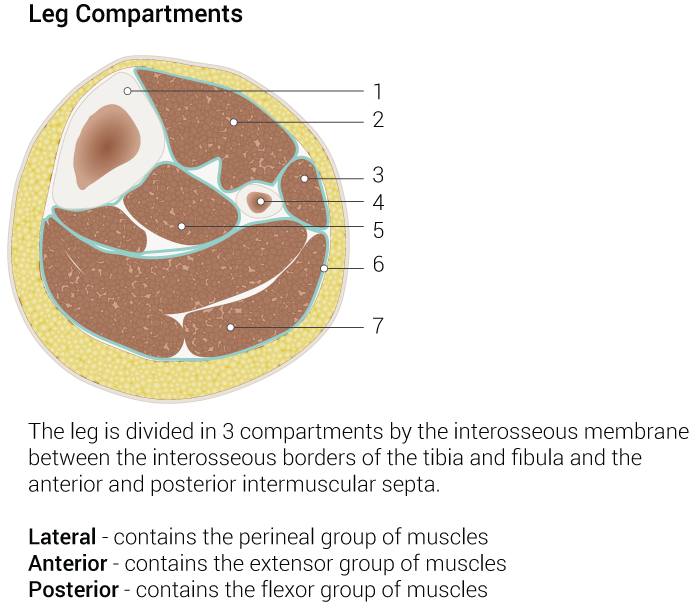
The fibula is a long, straight, and thick bone measuring approximately 3 x 40 cm, with a slightly greater length in males than females. This bone has a tricortical profile, meaning it has a triangular cross-section. The shape of the fibula is determined by the muscle attachments along its length, contributing to its overall structure and function within the lower limb. The mean fibula length is 387.4 ± 23.7 mm in males and 361.5 ± 12.3 mm in females (see Image. Leg Compartments).
Joints and Supporting Structures
The proximal tibiofibular joint, formed between the head of the fibula and the lateral condyle of the tibia, is supported by a capsule and anterior and posterior superior tibiofibular ligaments. The lateral collateral ligament and biceps femoris provide coronal support. At the same time, external rotation of the ankle induces slight movement at this joint.
The distal tibiofibular joint, known as the ankle syndesmosis, comprises the fibrous connection between the fibular notch and distal tibia, encompassing the interosseous membrane, anterior and posterior inferior tibiofibular ligaments, and inferior transverse tibiofibular ligaments. The integrity of the distal 5 cm of the fibula is crucial for ankle joint stability; any disruption to these syndesmotic structures may lead to ankle instability.
Vascular Anatomy
The blood supply of the fibula is essential for graft viability and union. Penetrating periosteal vessels supply the metaphysis and epiphysis, while nonpenetrating periosteal vessels supply the diaphysis of the fibula.[10] The foot's primary blood supply traverses through the anterior tibial, peroneal, and posterior tibial arteries. The anterior tibial artery supplies the epiphysis and proximal fibula, while the peroneal artery provides blood to the middle third.[11] Consequently, the peroneal artery and its paired venae comitantes serve as the pedicle for vascularized fibular flaps, with the peroneal artery typically measuring 2 to 3 mm in diameter, accompanied by veins measuring 3 to 4 mm wide.[12]
Understanding the anatomy of the fibula is crucial for determining the appropriate segment for a patient's reconstruction. The length of the vascular pedicle can extend up to 15 cm when harvesting the distal fibula, whereas it may be shorter when using more proximal bone. Furthermore, perforating vessels from the peroneal artery can support a skin paddle with a surface area of up to 10 x 20 cm, which is particularly beneficial for head and neck reconstruction. Notably, the skin in this paddle can regain sensation if the lateral sural cutaneous nerve in the flap is connected to a sensory nerve at the recipient site. Vascularized bone grafts offer advantages over nonvascularized grafts, including better tolerance to early mechanical loading and reduced risk of bone resorption and stress fractures, as they can remodel over time.
Muscular Anatomy
The vascular pedicle courses between the tibialis posterior muscle (anteriorly) and the flexor hallucis longus (posteriorly), while the skin perforators travel within or near the posterior intermuscular septum. The tibialis posterior muscle features chevron-shaped fibers and rests against the posterior surface of the interosseous membrane, inserting on both the fibula and tibia, whereas the flexor hallucis longus inserts solely on the fibula. Within the fascia, separating the tibialis posterior and flexor hallucis longus lie the peroneal artery and its accompanying veins. At the same time, deeper structures include the posterior tibial artery, vein, and the tibial nerve. The lateral compartment adjacent to the fibula houses the peroneus longus (laterally) and peroneus brevis (medially), bordered anteriorly by the anterior intermuscular septum and posteriorly by the posterior intermuscular septum. The superficial peroneal nerve innervates the peroneal muscles and courses along the anterior intermuscular septum, providing sensation to the anterolateral leg and dorsum of the foot, excluding the first web space.
Beyond the mentioned structures lie the extensor digitorum longus and anterior compartment, followed by the soleus muscle and posterior compartment, delineated by the anterior and posterior intermuscular septa. Along the anterior surface of the interosseous membrane run the anterior tibial artery and veins alongside the deep peroneal nerve, which innervates the extensor muscles and supplies sensation to the first web space. Both the superficial and deep peroneal nerves stem from the common peroneal nerve, a branch of the sciatic nerve that traverses obliquely over the lateral aspect of the fibular neck from superoposterior to anteroinferior, risking injury by an osteotomy made too superiorly on the fibula.
Indications
Vascularized free fibula flaps can be used in the following scenarios:
- Traumatic bony defects >6 cm in length [13]
- Bony defects after oncologic resection
- Resistant pseudarthrosis [14]
- Limb length discrepancy
- Chronic osteomyelitis with bone loss [15]
- Mandibular reconstruction [16]
- Vascularized epiphyseal transfer (for a growing child following tumor resection)[17]
- Osteonecrosis of the head of the humerus, the femur, or the mandible [18]
Contraindications
Free fibular flaps are contraindicated in the following scenarios:
- Peripheral vascular disease involving the lower extremities
- Hypoplastic anterior tibial artery
- Small skin paddle available for flaps
- Venous insufficiency
- Deep vein thrombosis
- History of contralateral lower extremity amputation
- Cardiopulmonary comorbidities that contraindicate long general anesthetics
Notably, advanced age is not in and of itself a contraindication to fibular transfer.[3]
Equipment
The following equipment is needed:
- #15 blade scalpel
- Monopolar and bipolar electrocautery
- Soft tissue set with Langenbeck retractors, periosteal elevators, forceps, hemostats, clamps, needle drivers, and Metzenbaum scissors
- Gigli saw, or reciprocating saw
- Plates, screws, and fixation hardware as necessary
- Screwdriver
- Drill
- Operating microscope
- Microvascular instrument set
- Hemoclips
- Doppler probe for vascular assessment
- Dermatome for harvesting a split-thickness skin graft to cover the skin paddle donor site, if necessary
- Appropriate sutures for closing the leg and the recipient site
- Suction drains
- Dressing supplies for the leg
- Tracheostomy set and tube (depending upon the location of the recipient site)
- Feeding tube (depending upon the location of the recipient site)
Personnel
A team consisting of the following personnel is required:
- Anesthesiology team
- Primary surgeon (plastic surgeon, orthopedic surgeon, head and neck surgeon, or oral surgeon)
- Surgical assistants
- Radiographic technician
- Circulating nurse
- Surgical technician
Preparation
Recipient site evaluation includes identifying appropriate vessels to perfuse the flap, determining appropriate patient positioning, and choosing the method of bone fixation. All types of implants should be available (eg, plates, screws, K-wires, external fixators, and intramedullary nails).
Before surgery, the donor site undergoes assessment, including evaluation of skin condition, muscle bulk, and vascular status. Documentation of the preoperative neurological assessment is essential due to the potential for transient postoperative peroneal nerve palsy. Adequate and independent pulses at both the dorsalis pedis and posterior tibial arteries should be confirmed, with any uncertainty prompting further investigation via Doppler ultrasonography. In some cases, computed tomography (CT) angiography or magnetic resonance angiography may be necessary to assess vascular status. If the peroneal artery is the primary blood supply to the foot, harvesting the fibula from that side is contraindicated. Conversely, inadequate blood flow in the peroneal artery also precludes fibular harvest. Patients with oral cavity cancer undergoing mandibular resection may exhibit risk factors such as alcohol consumption and tobacco use, increasing the risk of peripheral vasculopathy, which can contraindicate fibular harvest.
While a normal 3-vessel runoff to the foot isn't mandatory for fibular flap transfer, adequate foot perfusion without a patent peroneal artery, whether through the anterior or posterior tibial arteries, is imperative. For instance, if both the peroneal artery and the anterior tibial artery are patent while the posterior tibial artery is not, it is deemed safe to proceed with fibular harvest. Similarly, fibular harvesting is feasible if the peroneal artery and posterior tibial arteries are patent but the anterior tibial artery is not. However, fibular harvest is contraindicated if the anterior and posterior tibial arteries are patent while the peroneal artery is not. Additionally, if the tibial arteries are hypoplastic and the peroneal artery is the sole blood supply to the foot, termed "peronea magna," fibular harvesting is discouraged.
If vasculopathy contraindicates fibula transfer, other options include vascularized iliac crest and scapular bone flaps, which can accommodate dental implants.[19][20] Osteocutaneous radial forearm flaps are another potential source of vascularized bone. Although dental implants may also be placed into these flaps, the comparatively thin available bone makes them a second-line choice.[21][22]
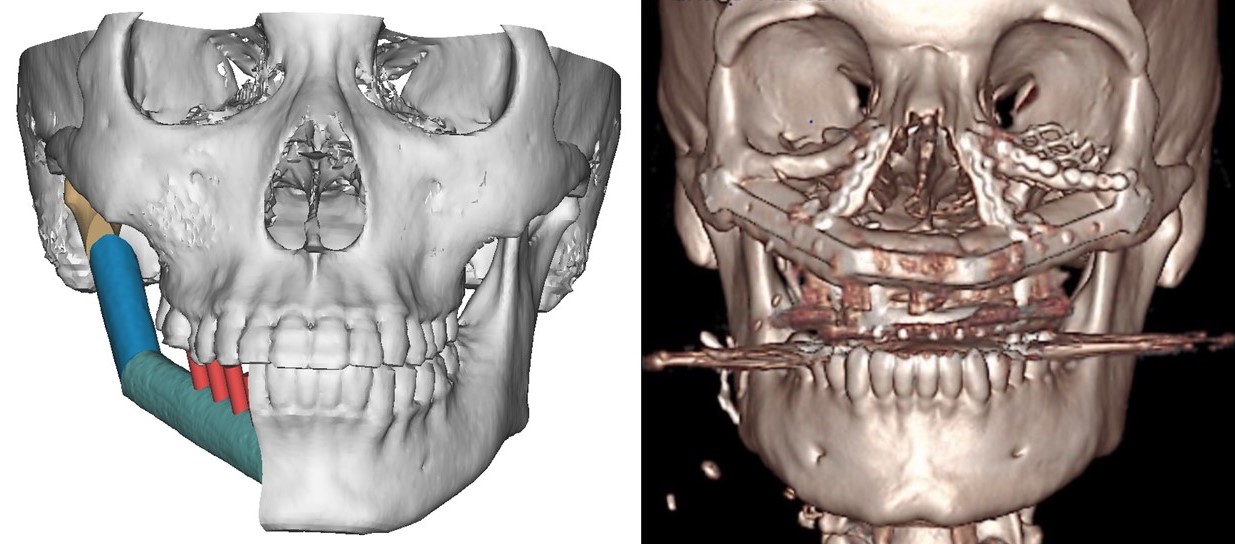
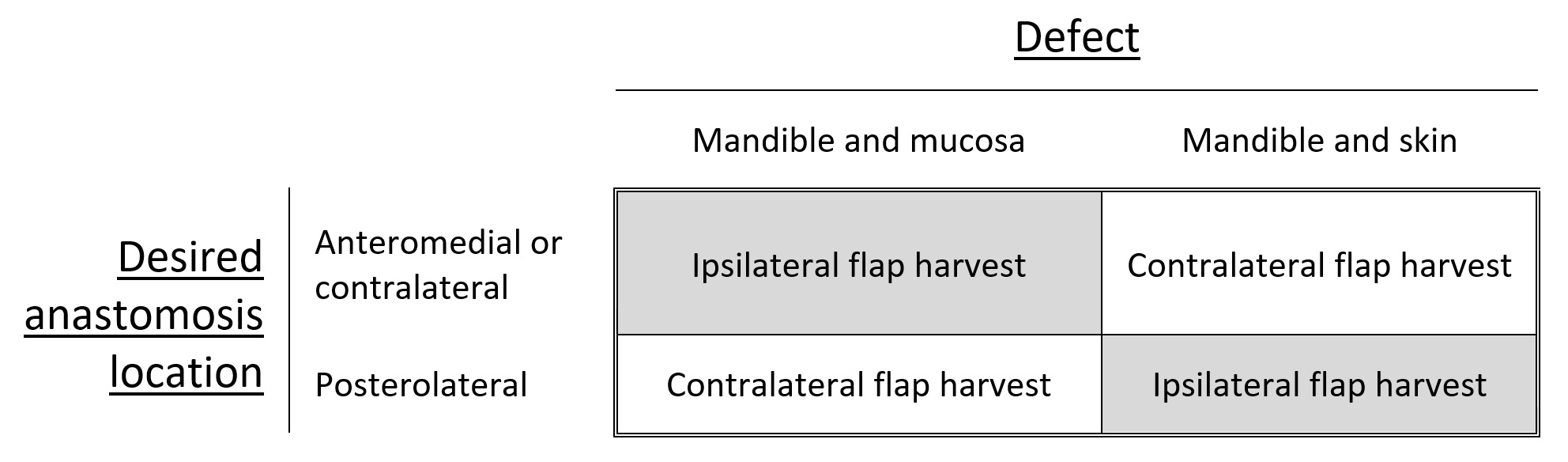
The contralateral fibula is preferred for tibial reconstruction, whereas femoral reconstruction commonly utilizes the ipsilateral fibula. Upper limb recipient sites may use either fibula. At the same time, selection for head and neck reconstruction depends on various factors such as neck vessel status, the specific area of mandibular or maxillary reconstruction, and whether the skin flap replaces oral mucosa or facial skin. In mandibular reconstruction, where mucosa replacement is necessary, a contralateral fibula is preferred to facilitate vascular anastomosis in the ipsilateral neck, often to facial vessels. Conversely, the ipsilateral fibula is preferred for maxillary reconstruction with a mucosal component. If vascular anastomosis needs to occur contralateral to the flap inset due to vessel depletion, the opposite fibula should be utilized (see Image. Fibula Flap Orientation for Mandibular Reconstruction).
Spatial planning is crucial in mandibular reconstruction with a fibular flap, encompassing decisions on which fibula to use, where to perform microvascular anastomosis, placement of closing ostectomies to conform the fibula to the mandibular contour, and positioning of dental implants if required concurrently with fibula transfer. Preoperative virtual surgical planning aids in these decisions, allowing manipulation of 3-dimensional (3D) renderings of the patient's face and leg CT images to simulate mandibular resection and fibula transfer, facilitating precise planning of osteotomies (see Image. Hemimandibulectomy Preoperative Planning and Reconstructed Maxilla Scan). Additionally, patient-specific cutting guides can be designed during planning sessions and 3D printed in polyamide or titanium for intraoperative use, ensuring optimal contouring when utilized with patient-specific 3D-printed titanium plates.[23][24]
Technique or Treatment
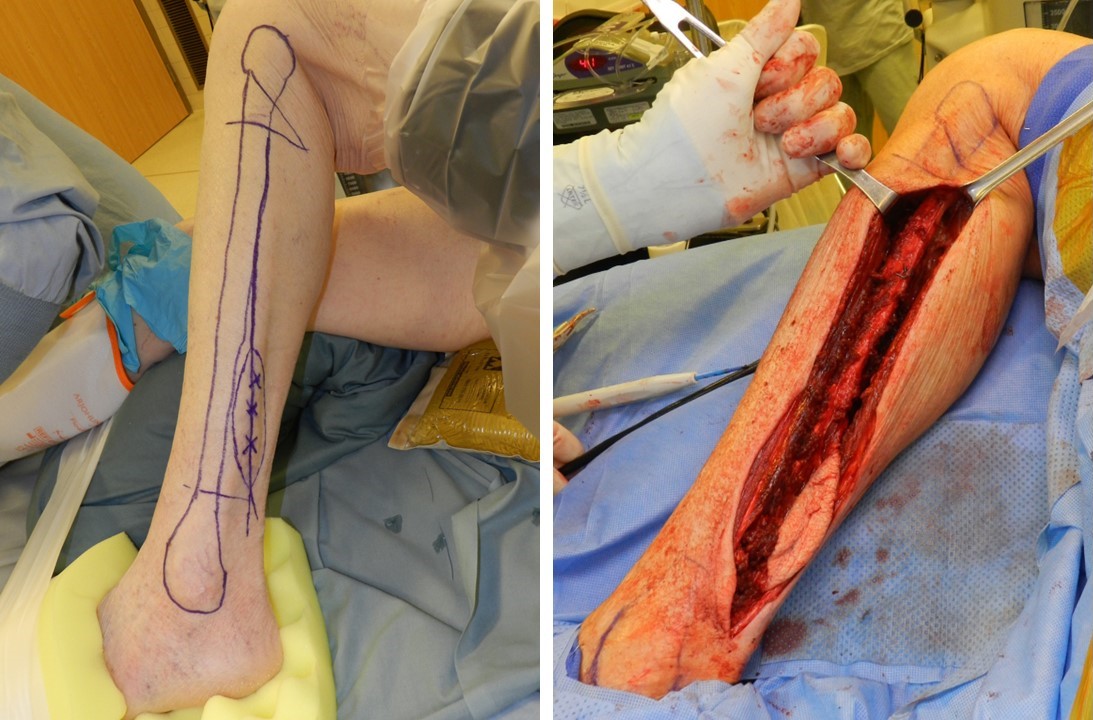
Following informed consent, prophylactic antibiotics are administered, and general anesthesia is induced. The knee is bent and pointed toward the ceiling to facilitate circumferential access to the lower leg. Subsequently, the fibula is outlined, and the skin paddle is marked, with 3 to 4 perforating vessels identified using a Doppler ultrasound probe (see Image. Preoperative Fibula Markings and Exposed Fibula). An Esmarch bandage is utilized to exsanguinate the leg, followed by applying a tourniquet to the thigh, inflated to either 150 mm Hg above systolic blood pressure or 350 mm Hg. The time that the tourniquet is inflated should be no more than 2 hours. All pressure points are then carefully padded due to the prolonged duration of the case. A Foley catheter and an arterial line may also be inserted for monitoring. Optimal efficiency is achieved by having 2 teams working simultaneously, 1 at the recipient site and the other at the harvest site.[25] Patient positioning is crucial to ensure both teams can work effectively, with adequate space for bringing the microscope close to the field for microvascular anastomosis.
Recipient Site Surgery
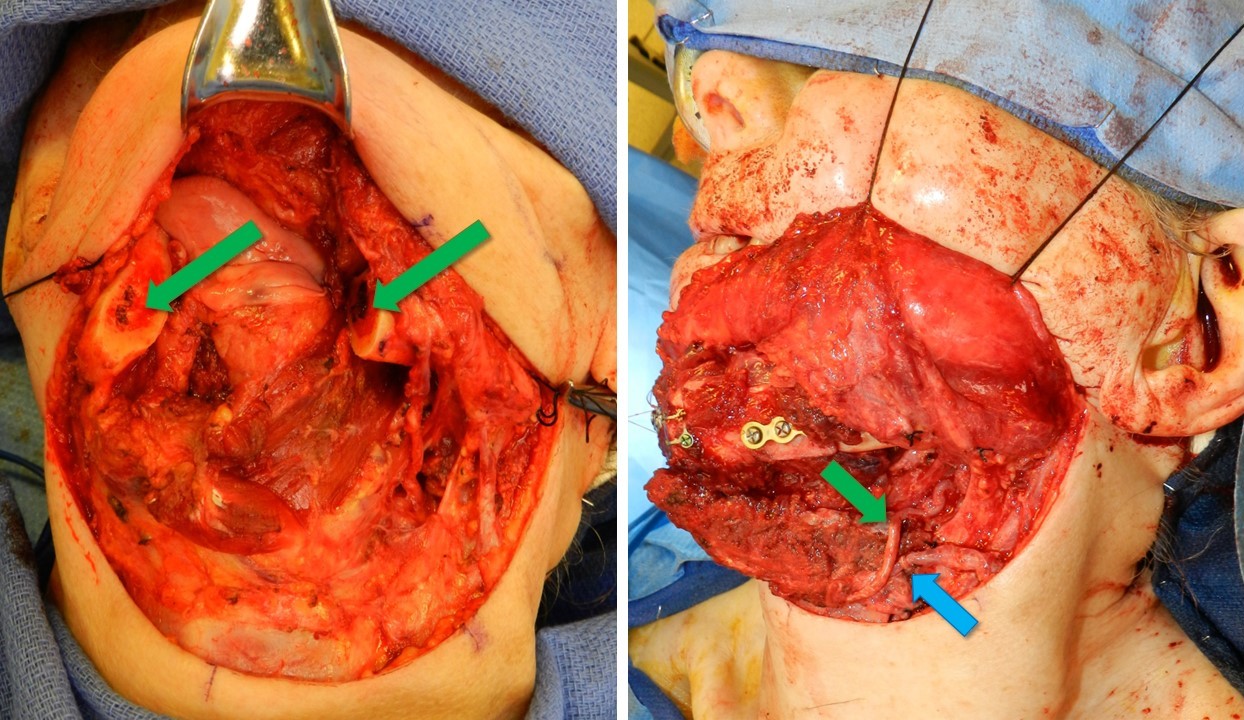
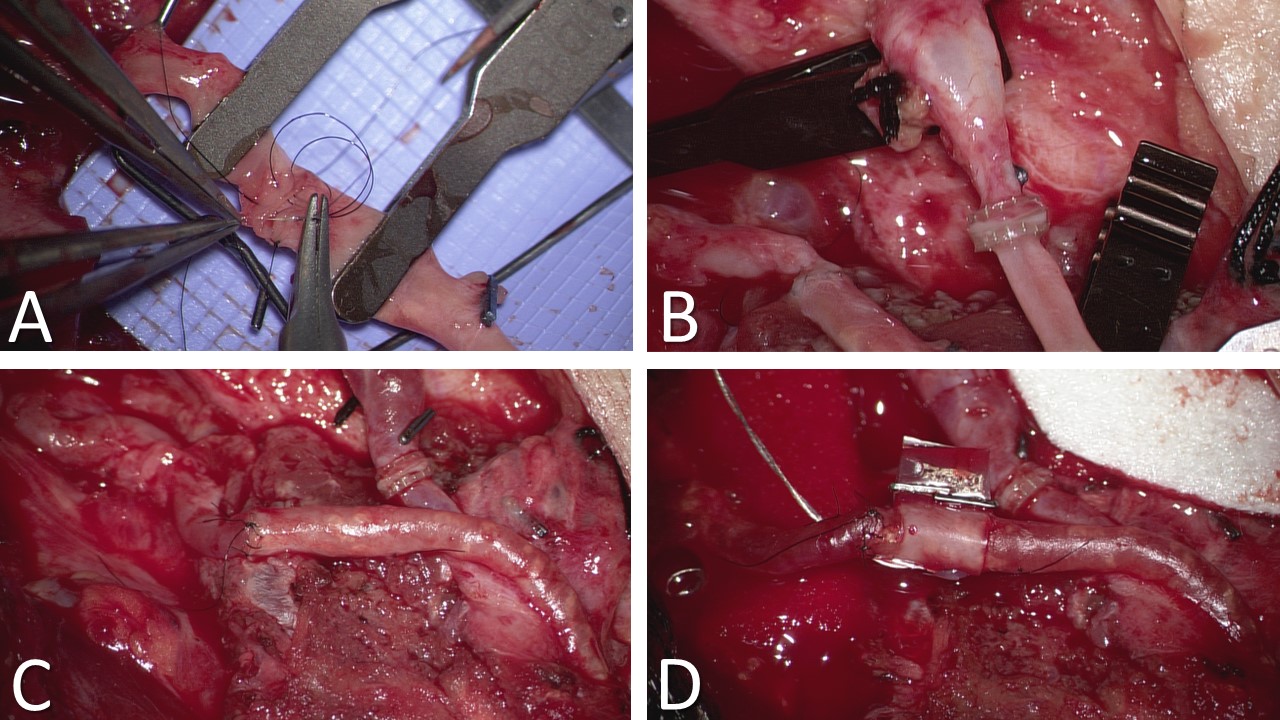
The surgical dissection at the recipient site is tailored to the specific diagnosis, dictating the extent of bone resection or debridement required. For instance, cases of pseudarthrosis may necessitate bone resection, while chronic osteomyelitis may require debridement and curettage. Reconstruction can be performed either as a single procedure or in 2 stages, such as in osteomyelitis (eradication of infection followed by free fibula transfer) or oncologic resection (ablation of tumor and lymph node dissection followed by fibula transfer after confirming negative margins). Selection and isolation of appropriate recipient vessels for vascular anastomosis are crucial, with communication of the defect size to the fibular harvest team to ensure an adequately sized graft.[26] In mandibular or maxillary reconstruction, the facial vessels are commonly utilized. However, other viable options include the superior thyroid artery, transverse cervical artery, superficial temporal artery, and external carotid artery (see Image. Vascular Anastomoses). The external jugular vein is a secondary option for venous outflow, with an end-to-side anastomosis into the internal jugular vein considered if other venous options are unavailable.
Donor Site Surgery
The lateral surgical approach is predominantly favored, involving a longitudinal incision along the lateral lower leg to expose the fibula (see Image. Preoperative Fibula Markings and Exposed Fibula). Typically, the central portion of the fibula is harvested, preserving the distal 5 cm for ankle joint stability and the proximal 5 cm to safeguard the common peroneal nerve. Proximal and distal bony cuts are carefully executed, with some surgeons opting to harvest the entire fibula length between the proximal and distal 5 cm. In contrast, others harvest only the required length for defect reconstruction. Preserving the peroneal artery and its venae comitantes is paramount to avoid injury. The distal fibula can provide up to 15 cm of vessel length for cases requiring a longer vascular pedicle. In some cases, a graft 2 to 3 cm longer than the bony defect may be required, as overlapping the graft on the osteotomy site may be necessary for fixation.[27] An example of the lateral approach to fibula harvest for head and neck reconstruction is detailed below:
The surgical procedure begins with an incision along the anterior aspect of the skin paddle, followed by meticulous dissection through the subcutaneous fat and fascia towards the fibula. Perforating blood vessels are identified and preserved without being skeletonized, often running through the soleus muscle or the posterior intermuscular septum. Dissection continues onto the lateral aspect of the fibula, separating the peroneus brevis to expose the entire lateral fibula. Anterior dissection proceeds until the anterior intermuscular septum is reached and divided, separated by the extensor digitorum longus muscle from the anterior fibula.
Continued dissection anteriorly encounters the interosseous membrane, which is carefully divided to avoid injury to the anterior tibial artery, veins, and deep peroneal nerve. Posteriorly, the incision is extended between the flexor hallucis longus and soleus muscles, preserving the previously identified perforating vessels—a cuff of soleus muscle with the flap aids in vessel protection. Incising along the flexor hallucis longus fascia allows the posterior tibial artery with its veins and the tibial nerve to retract from the surgical field, known as the O'Leary maneuver.
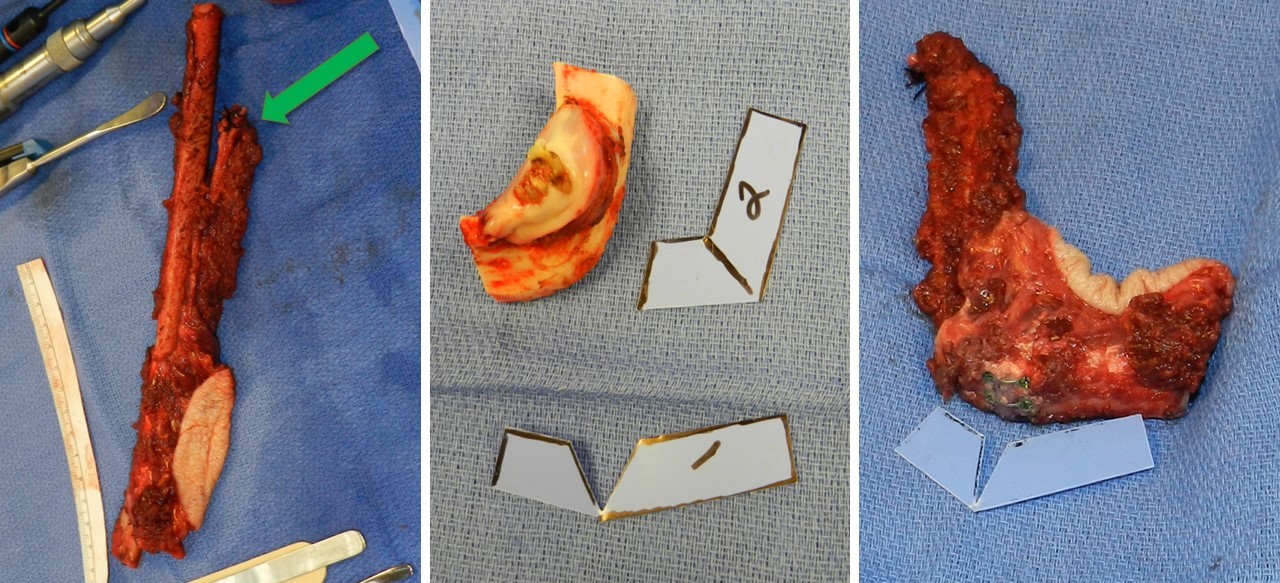
After blunt dissection with a large right-angle clamp close around the deep aspect of the superior fibula at the osteotomy site, a Gigli saw is used to make superior and inferior osteotomies over malleable retractors to safeguard underlying tissues. The fibula is distracted laterally to facilitate identification and ligation of the distal aspect of the vascular pedicle. The remaining muscular attachments on the fibula, such as the tibialis posterior and flexor hallucis longus, are divided before ligating the superior aspect of the pedicle (see Image. Fibula Free Flap Harvest and Closing Ostectomy). After deflating the tourniquet and achieving hemostasis, the donor site wound is closed layer by layer over a suction drain. Primary closure may be possible in narrow or nonexistent skin paddle cases, but a split-thickness skin graft is often required.
In select cases, a Langenskiöld procedure may be utilized prophylactically, involving fixation of the remaining distal fibula to the distal tibia to stabilize the ankle and prevent postoperative valgus deformity.
Graft Fixation
Various fixation techniques can be employed depending on the type of reconstruction needed. Options include intramedullary nails, compression plates, and the Ilizarov fixator. Hardware should not be changed once vascular anastomosis is complete, and care should be taken when placing the screws as they can compromise the blood supply to the graft. Typically, the graft is secured into the defect, and a portion of the soft tissue flap is sutured before anastomosis to ensure correct pedicle orientation, preventing vessel twisting or kinking during microsurgery. A 2 mm reconstruction bar is commonly utilized for mandibular reconstruction, sometimes supplemented with miniplates, especially for closing ostectomies. Closing ostectomies involves removing wedge-shaped bony segments to facilitate fibula bending and conformity to the mandible's shape, preserving periosteum to maintain perfusion (see Image. Resected Mandible and Fibula Free Flap Placement).
Often, excess fibula length or portions removed for contouring are repurposed. The excess bone may be milled into bone pâté or split into nonvascularized cortical grafts for additional reconstruction.[28] In cases where fibula diameter is insufficient, a "double-barreled" approach may be necessary, involving adjacent placement of 2 fibula segments while preserving periosteum to ensure blood flow. This technique is beneficial for enhancing height in maxillary reconstructions or width in femoral and tibial metaphyseal repairs.[29][30]
In mandibular reconstruction, a novel approach is the "jaw in a day" concept, involving dental implant placement into the fibula during flap transfer under the same anesthesia as bone harvesting. A provisional prosthesis is promptly fitted to the neomandible, significantly expediting oral feeding resumption for the patient.[31]
Additional Procedures
In complex head and neck reconstructions requiring extensive epithelial coverage beyond what an osteocutaneous fibular flap can provide, a fasciocutaneous radial forearm flap may be added. This flap can be anastomosed to different recipient vessels in the neck or incorporated in a "flow-through" configuration, connecting the proximal end of 1 flap's pedicle to the distal end of the other. When a skin paddle is harvested with the fibular flap or a radial forearm flap, skin grafts are typically needed to close the donor site defect(s), often sourced from the thigh using a powered dermatome.
Patients undergoing mandibular reconstruction, particularly those with a skin paddle, may experience significant intraoral edema, hindering oral feeding and breathing. These cases often necessitate a tracheostomy at the start of the operation and the placement of a nasogastric feeding tube at the end. For those seeking dental rehabilitation, implants can be placed in a subsequent procedure if a "jaw in a day" approach wasn't feasible initially.
Complications
The following are various complications related to fibula free flaps:
Anesthesia Related Complications
- Nausea and vomiting
- Dental injuries during intubation
- Sore throat
- Anaphylaxis to anesthetic agents
- Myocardial infarction
- Respiratory depression
- Aspiration pneumonia
- Hypothermia [32]
Intraoperative Complications
- Bleeding
- Length discrepancy of graft
- Iatrogenic graft fracture
- Improper fixation
- Damage to the neurovascular bundle [33]
Postoperative Complications
- Graft fracture (most common complication)
- Surgical site infection (second most common complication)[18]
- Prolonged hospital stay
- Great toe contracture (flexor hallucis longus lengthening usually corrects the contracture)
- Peroneal nerve injury [34]
- Hardware failures or extrusion
- Gait disturbance due to pain (generally less than that resulting from fibular fracture)[35]
- Nonunion/malunion
- Growth abnormality due to physis injury [25]
- Wound dehiscence
- Amputations [36]
- Leg length discrepancies
- Valgus malalignment of the donor limb
- Equinus deformity of the ankle [37]
- Nerve injury with sensory loss or motor deficit
- Chronic ankle pain
- Expanding hematoma
- Thrombosis of the anastomosed vessels and partial or complete flap failure
Clinical Significance
Autogenous vascularized fibular grafting is integral in reconstructing long bone defects, infected nonunions, and oncologic defects in adult and pediatric patients. These grafts offer osteoinductive and mechanical support and have a unique ability to remodel and hypertrophy, ensuring durability under loading conditions. This characteristic sets vascularized grafting apart from other forms of bony reconstruction. Achieving a successful outcome hinges on the care team's experience, meticulous patient selection, and effective preoperative expectation management.
Enhancing Healthcare Team Outcomes
In fibula free flap surgeries, a multidisciplinary approach involving physicians, advanced practitioners, nurses, pharmacists, and other health professionals is essential to ensure comprehensive patient-centered care, optimize outcomes, enhance patient safety, and improve team performance. Physicians, including surgeons specializing in reconstructive and microvascular surgery, play a central role in patient evaluation, surgical planning, and execution of the fibula free flap procedure. Their expertise is critical in determining patient candidacy, performing intricate microsurgical techniques, and overseeing postoperative care. Advanced practitioners, such as physician assistants and nurse practitioners, collaborate closely with physicians to provide preoperative assessments, assist in surgical procedures, and manage postoperative complications, contributing to efficient and effective care delivery.
Nurses are pivotal in the patient's surgical journey, from preoperative education and preparation to intraoperative monitoring and postoperative care. Their responsibilities include patient advocacy, symptom management, wound care, and patient and family education, ensuring a holistic approach to patient care. Pharmacists contribute by providing expertise in medication management, including perioperative antibiotics, pain management, and prophylaxis against thromboembolic events, thereby optimizing medication safety and efficacy. Effective interprofessional communication and collaboration are paramount to ensure seamless care transitions, timely interventions, and continuity of care. Regular interdisciplinary team meetings, clear communication protocols, and shared decision-making processes foster a culture of collaboration and enhance care coordination, ultimately leading to improved patient outcomes and enhanced team performance in fibula free flap surgeries.
Nursing, Allied Health, and Interprofessional Team Interventions
Attentive nursing care is critical to monitor for flap complications in the immediate postoperative period.
Physical therapists will help to mobilize the patient early, which can prevent such perioperative complications as deep vein thrombosis and pulmonary embolism.
In mandibular or maxillary reconstruction cases, speech and swallowing therapists and nutritionists are critical to maintaining the patient's caloric intake. Oral feeding can generally be resumed 2 to 3 weeks after facial reconstruction, and the tracheostomy can usually be removed 1 to 2 weeks postoperatively.
Nursing, Allied Health, and Interprofessional Team Monitoring
Monitoring the flap for signs of failure is critical in the first 2 weeks of healing, especially within the first 72 hours postsurgery when the anastomosed vessels are undergoing reepithelialization. Key indicators of flap failure include changes in color to a dusky or pale hue, decreased tissue temperature (except for intraoral flaps), soft tissue rigidity due to edema, either very rapid or absent capillary refill in the skin paddle, immediate or no return of blood when punctured with a lancet, and the absence of arterial Doppler signals. Often, loss of the Doppler signal is the last sign of a flap failure because venous congestion accounts for 80% to 90% of flap failures, and the arterial signal may not disappear until the flap is fully congested, which occurs very late in the process. In cases of vascular compromise, prompt return to the operating room for revision of the anastomoses is imperative.[38] Vascular compromise affects fewer than 10% of microvascular free flap cases, with salvage rates exceeding 50% if intervention occurs within 3 hours. Flap failures occurring within 24 to 48 hours postsurgery have a higher likelihood of successful salvage than those occurring later in the recovery period.[39][40][41]