Introduction
B cell or B lymphocyte (bursa-derived cells) is a key player of the adaptive immune response that is responsible for humoral immunity in mammals. B-cell production in humans is a lifelong process that starts in the fetal liver intrauterine and bone marrow after birth. Their development is from hematopoietic stem cells. B-cell development constitutes of all the stages of early differentiation in the absence of antigen interaction until the maturation, antigen interaction, and, ultimately, antibodies synthesis. By this process, B cells acquire two important features of adaptive immunity: (1) discrimination between self and non-self (the ability of B-cell to recognize foreign antigens rather than self-antigens) (2) memory (the ability to recall the previous contact with antigens, therefore, subsequent interaction leads to a more effective and quicker response).
B cells acquire their name from the early experiments on chicken that demonstrate the synthesis of antibodies. Max Cooper founds that antibody production in chicken requires an organ called the bursa of Fabricius in the 1960s. Antibody production was inhibited after the surgical removal of the bursa. The cells that are responsible for antibodies production were called bursa derived or B cells. In contrast to chicken, B-cell development in humans took place predominantly in the bone marrow. Many B-cell differentiation pathways demonstrate characteristic specific surface markers (CD markers) and immunoglobulin (Ig) gene arrangements. Additionally, developmental checkpoints exist along the pathways to determine whether the cell goes into the normal pathway or an alternative pathway resulting in cell death.
Issues of Concern
B lymphocytes originate in the bone marrow; their goal is to recognize an antigen. Each of them responds to the antigen in a specific way, as it expresses specific antibodies on the membrane (IgM, CD79a, and CD79b).
Structure
Unfortunately, few studies of the composition and the internal environment of b lymphocytes exist; consequently, this section will focus on b lymphocyte external surface and associated structures. B cells have a plasma membrane composed of equal parts of the weight of protein glycosphingolipids and carbohydrates. The upcoming paragraphs will describe the most critical external structures of B-cell responsible for cell activation, antigen recognition, and signal transduction.[1]
Stage-specific Markers
Different molecules are presented on B cells in various stages of development/maturation and activation. For example, CD10 is expressed on first-stage cells on B cell lineage like pro-B, pre-B cell, and germinal centers cells. CD19 and C20 are expressed on all cells of b cell lineage except plasma cells. However, CD27 is exclusively expressed on memory and plasma cells. Also, B-1 cells are characterized by the CD5 molecule.[2]
Antigen-binding Molecules (membrane immunoglobulin):
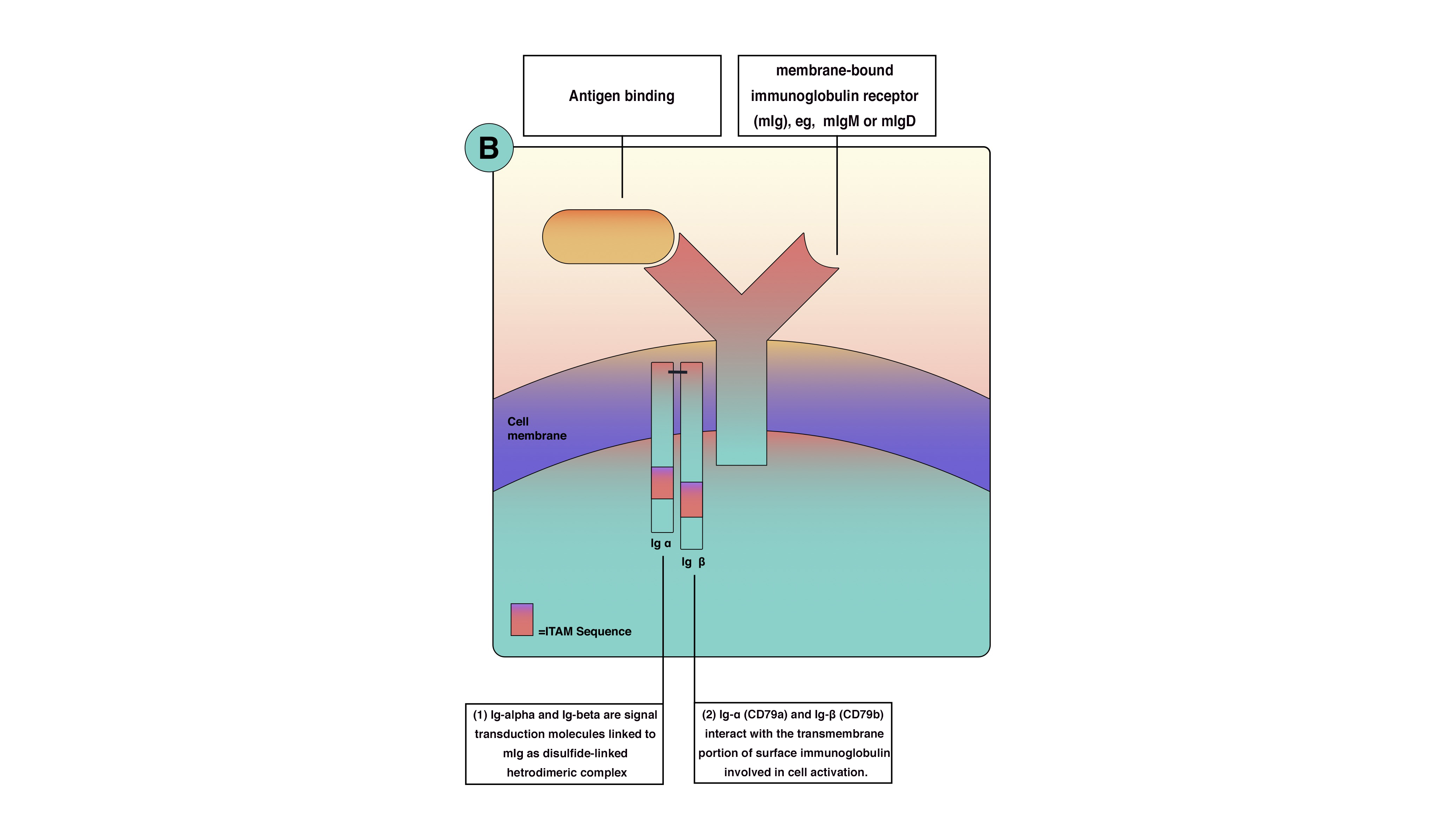
B cells antigen receptor is functionally part of multimolecular protein complexes at the cell surface. The b-cell antigen receptor is a transmembrane receptor that extends to the cytoplasm. However, it has very short cytoplasmic sequences (tails). These tails are ineffective in transmitting the signals and activating B cells. Consequently, another protein is involved in the process of signal transmission and lymphocyte activation. B cell receptor(BCR) is a multimolecular protein complex bounded noncovalently with other proteins. BCR is functionally involve: (1) membrane-bound immunoglobulin receptor (mIg), Ig-alpha (CD79a) and Ig-beta (CD79b). Ig-alpha and Ig-beta are signal transduction molecules that link to mIg as a disulfide-linked heterodimeric complex. Ig-alpha and Ig-beta contain a sequence called immune receptor tyrosine-based activation motif (ITAM) essential for signal transduction in both B and T lymphocytes. ITAM is responsible for passing the activation signals from the cell surface to the cytoplasm through tyrosine amino acid, which becomes phosphorylated by protein tyrosine kinases(PTKs) during cellular activation to interact with cytoplasmic signaling proteins. The thinking is that there is enough B cell receptor antigen for every microbe because of genetic arrangement and diversity. B cells receptors are inherited as gene fragments; these fragments are joined differently in each developing cell to create a diverse array of receptors. Theoretically, b lymphocyte can make up to 10^11 different antibodies in an individual. Besides, B cells receptors undergo somatic hypermutation create unique receptors.[3][1][4][2][5]
Co-receptor Molecules of B Cells
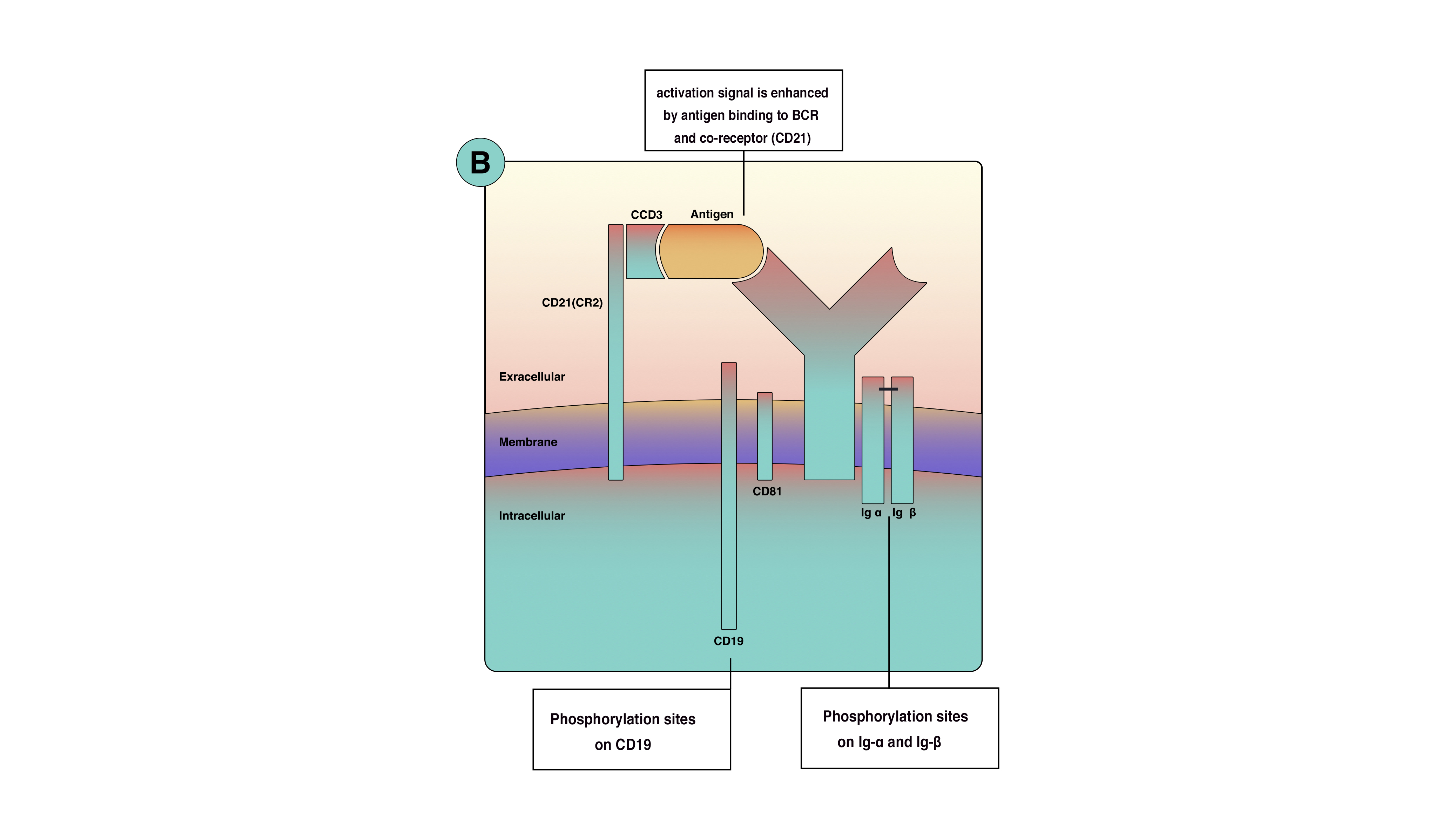
Optimal signaling in B-cell activation requires more than BCR, so co-receptors appear on the cell membrane. B cells co-receptors are clusters of molecules that increase the efficacy of signaling up to a thousand folds. B cells co-receptors are proteins known as CD21(also known as complement receptor 2); CD19 CD81 and CD225. These proteins are close to BCR but are not part of it. Phosphorylation of co-receptors proteins along with Ig-alpha and beta will amplify the activation singles from the cell surface to the cytoplasm as shown in (figure 1)Moreover, co-receptors reduce the BCR stimulation threshold, which means fewer antigens are necessary for BCR stimulation. Co receptor function is best demonstrated when microbial pathogens activate complement and then binds to B-cell. When antigen binds to complement protein C3d, this process will allow the antigen to bind to CD21 and BCR simultaneously to unable co-receptors complex to cluster and cross-link with BCR and phosphorylate CD19 tail (figure 2). This process will increase signals concentration around BCR.[2]
Signal Transduction Molecules (Molecule involved in T-B cells interaction)
In Thyms dependent B cells activation, T-B cells interaction occur that requires surface molecules; these molecules are:
- Major histocompatibility complex class II molecule: these present molecules peptides drove from T dependent protein antigens to T helper cells (CD4+). All B cell linage cells express MHC II except pro-B cells.[6]
- Co-stimulator molecules:These molecules are required as a second signal to accompany the first signal after antigen binding. Co-stimulators are expressed in high quantity in the activated cells rather than naïve B-cells. Several co-stimulatory have been studied; the most well know are B7 and CD40. B7 is a family of different molecules that interact with CD28 on T-cells surface. CD40 on B-cell interact with CD40 ligand (CD40L or CD154) on activated T-cells. This interaction is crucial in somatic hypermutation and class switch. Moreover, inducible co-stimulatory ligand on B cells interacts with ICOS on activated T-cells, which is critical and necessary for germinal center formation. Therefore, People who lack functional ICOSL or ICOS make deficient levels of IgG, IgA, IgE.[2]
- Cytokines receptors: cytokines produced by activated T lymphocytes (CD4+) mediate B cell response to protein antigens. CD40 ligand on the surface of TH lymphocytes interacts with CD40 molecule and functions to allow B lymphocyte development into antibody-secreting plasma cells.[2]
Function
Generally, B-cell is a key regulatory cell in the immune system; it acts by producing antibodies, antigen-presenting cells, supporting other mononuclear cells, and contributing to inflammatory pathways directly. However, to understand the nature, function, and subsequent dysfunction of b-cells, b-cell development will be discussed briefly.
B lymphocytes arise from hematopoietic stem cells that are considered as the precursor of B-cell lineage. Early development took place in fetal liver and bone marrow after birth, and throughout life hence bone marrow is the primary lymphoid organ in humans and many mammals. B-cell's continuous differentiation ensures that B cell repertoires are continuously replenished for limitless antigens recognitions. Non-lymphoid cells called stroma make up the matrix of the bone marrow providing essential molecules for cell production like Interleukin (IL-7), cytokines, and adhesion molecules that are critical for B-cell survival and differentiation. Major developmental stages of B cells result in cells that undergo a negative selection process to eliminate self-reactive cells to prevent autoimmunity. Those B cells which survive negative selection will be carried through the circulation to peripheral lymphoid organs waiting for antigens to react against and ultimately become antibody-secreting cells or plasma cells. However, B cells undergo program cell death if it did not encounter antigens.[7]
Tolerance to self-antigens must include all self-antigens. However, not all self-antigens present in the bone marrow. Therefore, another tolerance mechanism ensures that B cells do not cause autoimmunity. Generally, mature B cells require T cells help to produce antibodies. B cells that encounter antigen and do not receive help from T helper cells that are specific to that particular antigen will undergo anergy or clonal deletion. However, a subset of mature B cells have developed another mechanism to respond to antigens without the help of B cells; this population is called thymus independent B cells (T-independent B cells).[7]
Lymphoid follicles present in secondary lymphoid organs and provide a specialized environment to concentrate antigen for proper B cells function. It contains follicular dendritic cells to display antigens to naïve B cells. Secondary lymphoid tissue trapped antigens from different sources according to their location and related environment, (1) spleen collects blood-borne antigens, (2) lymphatic nodes collects antigen trapped in the lymphatic system, (3) mucosa-associated lymphoid tissue (MALT) acquires antigens from the surrounding mucosal epithelium.[8][9]
B cells are responsible for mediating the production of antigen-specific immunoglobulin (Ig) directed against invasive pathogens (antibodies). B cells recognize antigens via a membrane-bound b-cell receptor (BCR) along with accessory cell surface receptors. B cells are capable of recognizing a variety of structural motifs (epitopes) on antigens that rely on the enormous sequence and structural diversity of BCR repertoires, due to Genetic rearrangement in V(D)J segments responsible of the variable regions (heavy and light chains) of BCR. Stimulated B cells mature into plasma cells after antigen stimulation leading to the synthesis of five different antibody immunoglobulins classes that synthesize large amounts of antibody Immunoglobulins (IgA, IgG, IgD, IgM, and IgE). Once activated, the B cell undergoes mitotic division after activation to produce a clone of cells that the ability to produce immunoglobulin of the same antigen specificity. Theses cells will mature mostly into plasma cells. The primary immune response is generated when B-cell encounters antigens for the first time. However, a few subsets of this clone will mature into memory cells that respond rapidly upon subsequent exposures to that particular antigen and generate a secondary immune response. the secondary immune response is of high magnitude, occurs much more rapidly, and produces IgG rather than IgM. This phenomenon is the key concept om lifetime immunity and vaccines.[10][3][11][12][13][4]
In addition to their crucial role in humoral immunity, B cells also mediate/regulate many other functions essential for immune homeostasis. Experimental studies showed that depletion of B-cells during mice development leads to severe consequences and congenital abnormalities within the immune system (etc., generalized decrease in number and diversity of T-cells, an absence of Peyer patch organogenesis and defects within dendritic cells). Moreover, B-cell is necessary for immune system maintenance. For example, b-cell releases immunomodulatory cytokines that influence immune cells function like T-cells and dendritic cells, regulate lymphoid tissue organogenesis, wound healing, and transplanted tissue rejection. Furthermore, regulatory B-cell has been discovered as a critical cell that regulates T cell-mediated inflammatory responses by producing IL-10.[11][14]
B cells proliferate rapidly after antigenic stimulation in germinal centers rating about one division every 6 hours. Germinal center is a lightly stained region within lymphoid follicles made up of FDCs. During the proliferation process, point mutations are introduced at a high rate without repair into immunoglobulin genes; this unique process is referred to as somatic hypermutation. Moreover, Ig class switching is a crucial step in B-cell development in the germinal center, up to the end of B cell maturation it has IgM and IgD surface immunoglobulins but only capable of secret IgM. In many situations like mucosal infection were IgA is the cornerstone in fighting antigens, IgM is not enough; therefore, class switching is necessary to allow B cells to secret all classes of antibodies. Ultimately mature B cells encounter antigens and differentiate into plasma cells that secrets large amounts of antibodies and memory cells that rapidly respond to antigens upon subsequent exposers.[10][3][11][12][13]
B-cell Activation
Activation of a B cell by a protein antigen requires B cell to function as an APC, presenting the protein epitopes on MHC II to helper T cells hence the name T- cell-dependent activation is given to this mechanism. However, polysaccharides, lipopolysaccharides, and other non-protein antigens are considered as T-independent antigens because they can activate B cells without antigen processing and presentation to T cells.
- T Cell-independent activation of B cells:
- This type of activation happens when B cells interact with T-independent antigens—the activation process composed of two signals. The first signal is, cross-linkage of multiple BCRS with repetitive epitope unites on the antigen surface. The second signal is the interaction of toll-like receptors with PAMPs or interactions with factors from the complement system. After B cell activation, B cells undergo clonal proliferation and ultimate differentiation of daughter cells into plasma cells. Ultimately, B-cell receptors will disappear. However, plasma cells will dominate in antibodies production of IgM type with the same specificity as BCRs (Pentameric IgM). This process is of short life and its lakes the ability of memory cell production.
- T Cell-Dependent Activation of B cells:
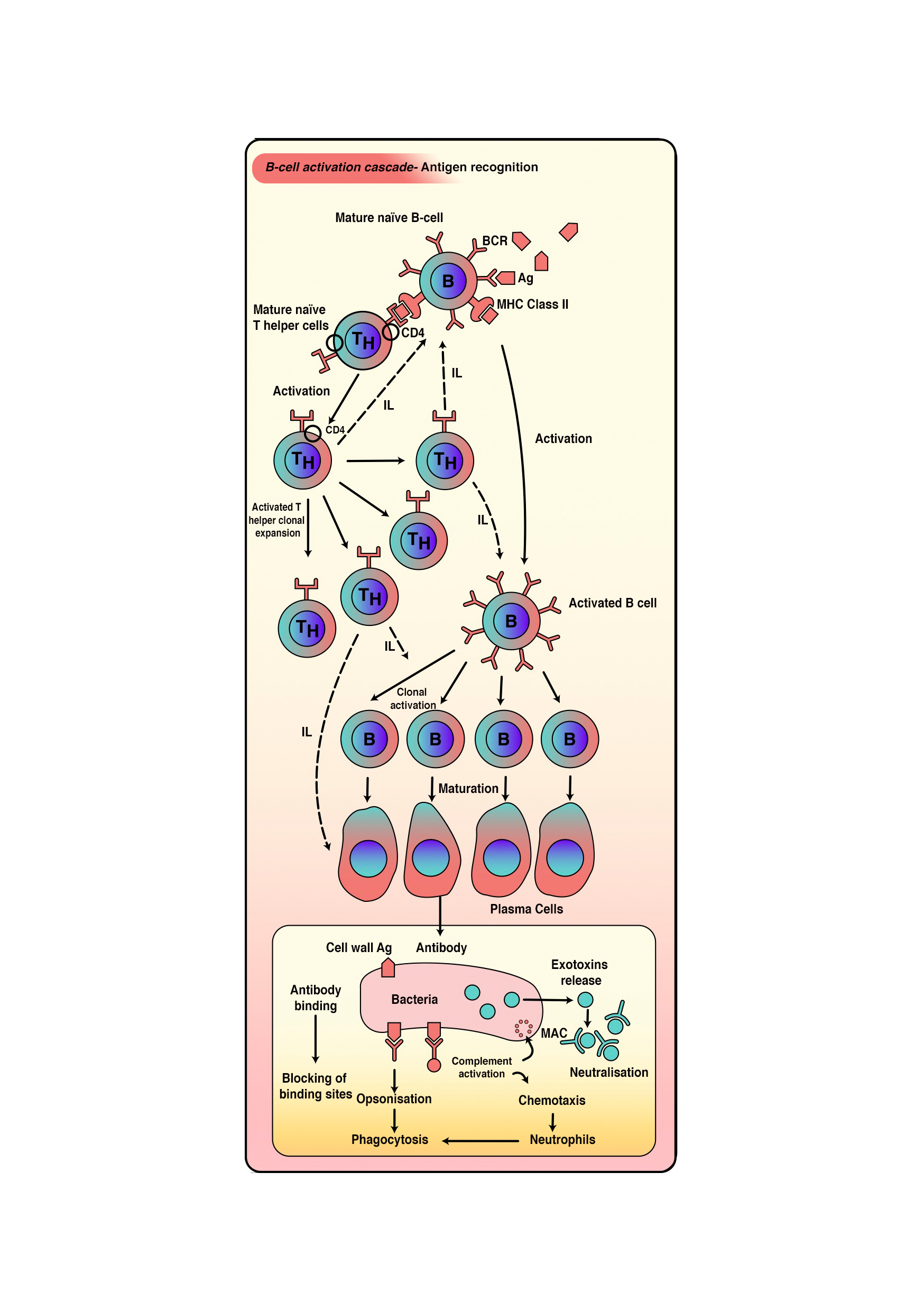
- This process happens in response to T-dependent antigens (protein material either free or associated with intact pathogens). Free antigen interaction will result in internalization directly. However, interaction with intact pathogens will result in antigen separation and extraction from the intact pathogen. Eventually, the antigen will be presented on MHC class 2 on B-cell's external membrane that is recognized by T helper cells specific to that antigen (figure 3). Linked recognition between T helper cells and B cells will happen, which explained as TCR of T helper cells recognizes the antigen presented on b cells and CD4 molecule interaction with MHC-II on B cells. Several cytokines secreted by TH2 cells will stimulate B cells to proliferate and differentiate into plasma cells and memory cells.[9][11][12][14][13][8]
Tissue Preparation
Normally, lymphocytes specimens are separated from blood samples by centrifuging. ultimately, samples are prepared in a specific way according to the studying method, refer to light and electron microscopy for further information.[15]
Microscopy, Light
Generally speaking, lymphocyte types are best differentiated through histochemistry and flow cytometry; the light microscope cannot differentiate between lymphocytes types based on appearance. However, light microscopy shows lymphocytes as spherical or ovoid cells that have diameters from 6 to 15 μm when flattened on glass slides. Sample preparation of lymphocytes usually requires staining by Romanowsky polychromatic stains (e.g., Giemsa or Wright) of air-dried films.
Light microscopy shows two populations of lymphocytes: (1) large cells with diameters of 9 to 15 μm and (2) small lymphocytes with diameters of 6 to 9 μm. Under the microscope, lymphocytes appear as dark purple cells with a deep bluish nucleus and faint sky blue cytoplasm. Nuclei are taking a large proportion of the internal environment of the cell due to a large amount of condensed chromatin.[15][16]
Microscopy, Electron
B lymphocytes can be studied using scanning electron microscopy (SEM) and Transmission electron microscopy (TEM). Yet, each method shows b cells from a different perspective. TEM allows us to look to the inside of cells. However, SEC shows the outer surface of B lymphocytes.
Transmission Electron Microscopy
Blood lymphocyte nucleus under TEM has electron-dense heterochromatin, which considered as a feature of Nonproliferating cells. Lymphocytes nucleoli are round in section. Lymphocytes are arranged concentrically into three zones or structural units. (1) the central region (the Agranular zone); (2) the middle (fibrillar region); (3) the outer (granular zone) that is composed of intranuclear chromatin. Furthermore, The lymphocytes cytoplasmic organelles are typical of eukaryotic cells.
Scanning Electron Microscopy
This modality provides three-dimensional information. However, it creates images with less resolution compared to transmission electron microscopy. Normal blood lymphocytes washed and collected on silver membranes and fixed in glutaraldehyde. B lymphocytes under SEM range from 5.1 to 6.4 μm in dimeter. B cells were identified by their complex surface architecture with multiple finger-like microvilli covering the entire surface of B cells.[15][16][17]
Pathophysiology
Studies show that intense physical exertion, such as extreme sports or alteration of gravity (space), reduces the amount of type B lymphocytes. This decline could be the cause of a physiological alteration of the immune response. Furthermore, it is necessary to consider the circadian rhythms of the production of these cells; external stress that alters the body's circadian status could negatively affect the immune response.
Clinical Significance
B-cell dysfunction is pronounced in several disorders included immunodeficiency, autoimmune diseases, and malignancies. As described earlier, B-cell is responsible for antibodies production, antigen presentation, and immune system regulation, and maintanceAccumulating evidence of several decades’ studies showed that disruption of any of these tightly regulated and controlled pathways might lead to autoimmunity, malignancy, or other well-known diseases. A fundamental feature of autoimmunity is the inappropriate production of autoantibodies and loss of b-cells tolerance. Genetic mutations in b cell lineage may lead to intrinsic disorders in b cells and consequent induction of autoimmunity in the T-cell compartment. Therefore, these findings showed the rationale of B cells in depleting b cells as a therapeutic strategy in autoimmune disorders and other diseases. The upcoming paragraphs show the role of B-cells in the development or initiation of certain disorders.[18][19]
Primary B-cell Immunodeficiencies or Pure B-cell Immunodeficiencies
This category refers to diseases resulting from abnormal B-cells function that includes antibodies production and B cell interaction with T-cells. Classically, patients present with recurrent infections and other complications depending on the disorder and the developmental stage in which the disorder has occurred: the earlier the defect, the more devastating the effect on lymphopoiesis. As b cells begin to express surface receptors, they are subjective to positive and negative selection pressure and become dependent on survival signals. Therefore, defect in these processes will lead to selective or generalized hypogammaglobulinemia.
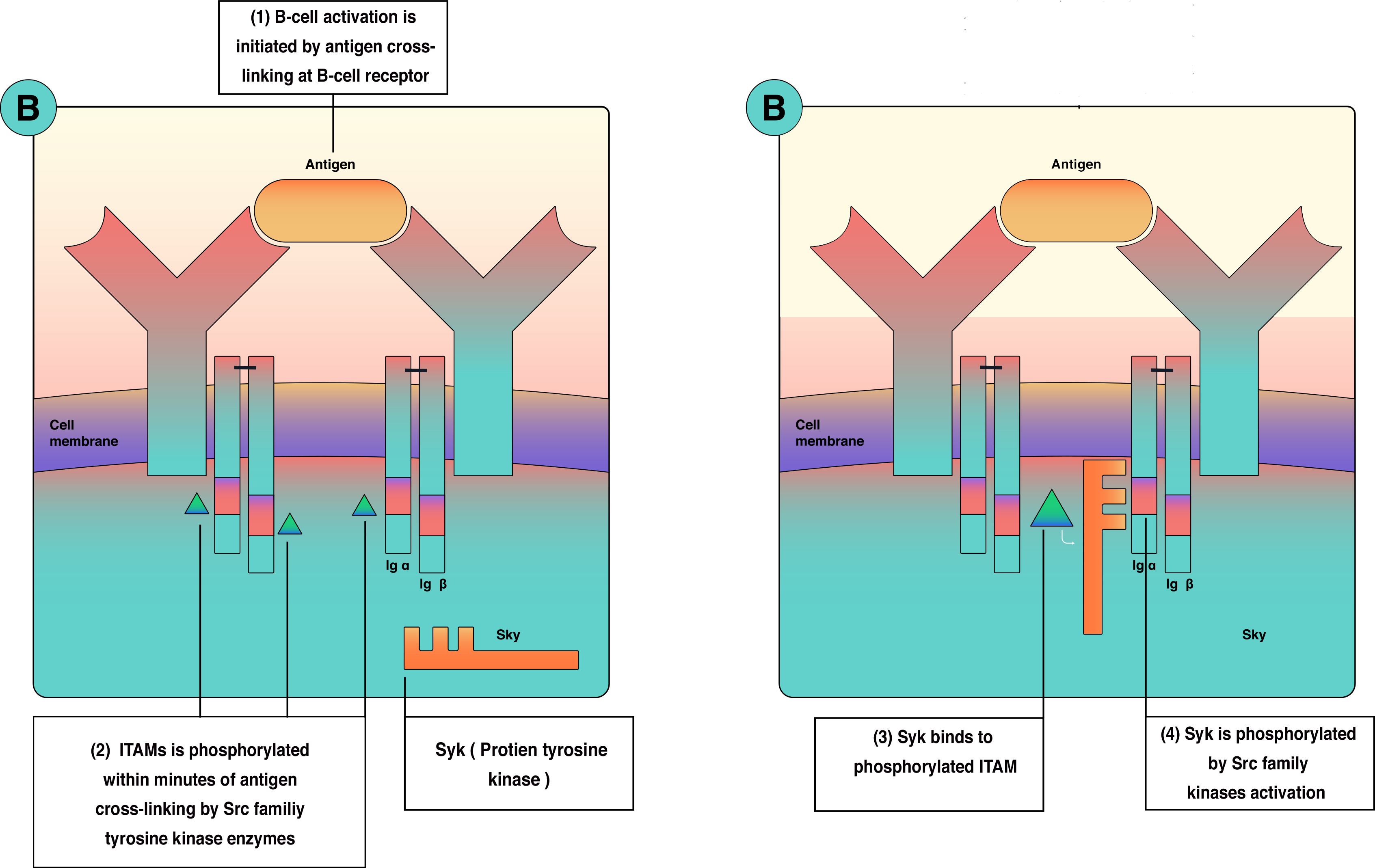
As discussed in the physiology section, b cell activation requires two waves of activation signals, the first one involves Src family and the second one involves Bruton tyrosine kinase (BTK) and Syk (figure 4). As a consequence, a mutation in the BTK gene will lead to X-linked agammaglobulinemia (XLA). Moreover, A failure to express CD40 ligand due to mutation in gp39 (CD40 ligand results in an inability to isotype class switch, causing X-linked immunodeficiency with hyper-IgM (XHM) that correlates in some patients with liver disease, sclerosing cholangitis, and liver/GI malignancies.[3][20][19][21]
Transplantation Associated B-cells Disorders
It is well-known that T-cells play a critical role in the development of chronic graft-versus-host diseases (GVHDs); nevertheless, studies showed that B-cells could participate in the pathophysiology through many effector pathways including, antigen presentation to T-cells and dysregulated autoimmune antibody synthesis.[22]
Autoimmune Diseases Associated with B-cells Dysfunction
Autoimmune disorders where disease activity correlates with b cell function include Multiple sclerosis, systemic lupus erythematosus (SLE), type 1 diabetes, scleroderma, rheumatoid arthritis, and post-infectious IBS. [23]
B-cell Malignancies
Malignant transformation of any step of B-cell lineage causes a host of cancers, including hairy cell leukemia, chronic lymphocytic leukemia, acute lymphoblastic leukemia, follicular lymphoma, Hodgkin’s and non-Hodgkin’s lymphoma and plasma cell cancers such as multiple myeloma.[18][24]