Continuing Education Activity
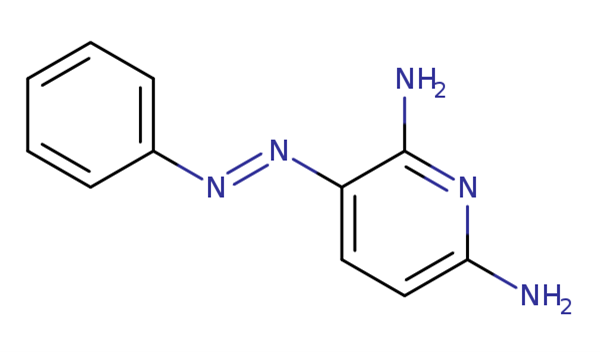
Phenazopyridine, an azo dye, is a urinary analgesic used as an adjuvant medicine in the outpatient setting to treat patients with urinary tract infections (UTIs) and related conditions (see Image. Phenazopyridine Chemical Structure). Phenazopyridine is approved by the US Food and Drug Administration (FDA) to alleviate symptoms of dysuria, burning sensations, urgency, frequency, and pain linked to lower UTIs. Phenazopyridine is a readily accessible medication that can be obtained over the counter (OTC) and by prescription (see Image. Over-the-Counter Phenazopyridine US FDA Labeling). Owing to its analgesic properties, phenazopyridine can be administered concurrently with antibiotic therapy to relieve patients' pain and enhance their comfort while waiting for effective infection management.
This activity reviews phenazopyridine's utilization, mechanism of action, potential adverse effects, off-label applications, dosing, pharmacokinetics, monitoring, and pertinent drug interactions. All healthcare team members involved in the care continuum of patients with UTI and associated disorders can benefit from this activity.
Objectives:
Identify patients with lower urinary tract infections who could benefit from phenazopyridine as an adjuvant treatment.
Screen patients for contraindications such as severe renal insufficiency and G6PD deficiency before prescribing phenazopyridine.
Implement accurate dosing regimens, aligning phenazopyridine usage with recommended guidelines and patient profiles.
Assess and evaluate patients for potential adverse effects related to phenazopyridine therapy, including urine discoloration and systemic reactions.
Indications
Phenazopyridine, an azo dye, is a urinary analgesic used as an adjuvant medicine in the outpatient setting to treat patients with urinary tract infections (UTIs) and related conditions. This medication is approved by the US Food and Drug Administration (FDA). It is used in an outpatient setting to treat patients with dysuria, burning sensations, urgency, frequency, and pain associated with lower UTIs.
Owing to its analgesic properties, phenazopyridine can be administered concurrently with antibiotic therapy to relieve patients' pain and enhance their comfort while waiting for effective infection management. Phenazopyridine's analgesic properties can reduce the need for other medications and systemic analgesics. In clinical and inpatient settings, phenazopyridine effectively alleviates trauma, catheter insertions, and surgical interventions. However, the drug's diagnostic applications are confined in scope.
History of Phenazopyridine
Phenazopyridine was initially synthesized in 1914 and later adopted by the US Pharmacopoeia in 1928.[1][2] Regulatory mandates did not necessitate preclinical studies to demonstrate the safety and efficacy of medications. Phenazopyridine was commonly prescribed for treating lower UTIs in patients under the mistaken belief that it had bactericidal properties. With the advent of antibiotics in the late 1930s, a definitive curative approach for UTIs was introduced, while phenazopyridine persisted as an adjuvant.[1] Over the decades, phenazopyridine has been marketed as a standalone drug or combined with antibiotics or other agents.[3]
Mechanism of Action
Although the mechanism of action of phenazopyridine lacks definitive substantiation, it is hypothesized that the drug exerts a localized analgesic effect on the urinary tract mucosa.[1][4] Evidence indicates that the drug inhibits nerve fibers in the bladder that respond to mechanical stimuli.[5] The drug also hinders kinases responsible for cell growth, metabolism, and nociception.[6]
Pharmacokinetics
While the pharmacokinetic characteristics of phenazopyridine are recognized, a comprehensive evaluation of these characteristics is lacking. These characteristics include the following:
Absorption: Following oral administration, the drug undergoes rapid absorption in the gastrointestinal tract and reaches a plasma time to peak drug concentration (Tmax) within 2 to 3 hours.[7][8] Although the exact absorption site of the drug is unknown, it is believed that there are 2 distinct regions involved.[7] Studies investigating the half-life of phenazopyridine have yielded inconsistent findings, with 1 study reporting an average half-life of 9.4 hours.[8] The extent of protein binding is also unknown.
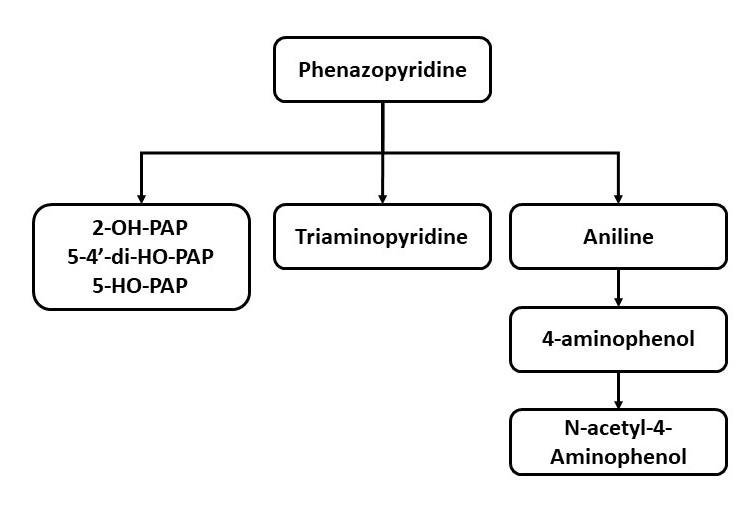
Metabolism: Information about the drug's metabolism is derived from animal studies.[9] Aniline and tri-aminopyridine are the 2 metabolites that could potentially be attributed to hematological and renal adverse events of the drug, respectively.[10][11] Another metabolite of aniline, N-acetyl-4-aminophenol, is commonly recognized as acetaminophen and paracetamol. Nonetheless, this metabolite's clinical relevance is considered negligible (see Image. Phenazopyridine Metabolism).
Elimination: Research indicates that after administering an oral dose of phenazopyridine, approximately 41% to 65% of the medication is eliminated unchanged by the kidneys.[10][9]
Administration
Adult Dosage, Dosage Forms, and Strengths
Typically, phenazopyridine is prescribed at a dosage of 100 to 200 mg 3 times daily for healthy adults. Over-the-counter (OTC) tablets are obtainable in lower strengths, ranging from 50 to 99.5 mg. The OTC tablets are frequently taken 2 at a time, 3 times daily, effectively matching the prescription regimen. The OTC formulation is a valuable option for temporary symptom relief while awaiting additional medical attention. To prevent stomach discomfort, phenazopyridine is advised to be taken with or after meals.[12]
The recommended phenazopyridine treatment duration is 2 days, and this short period provides swift relief from discomfort until antibiotics can effectively manage the infection and safeguard the patient from potentially severe adverse effects. Certain clinicians recommend that individuals experiencing uncomplicated UTI symptoms take OTC phenazopyridine alone for 5 days, expecting the infection to resolve spontaneously. However, most patients favor a more assured resolution, and while the practice is not yet substantiated, it is noteworthy that a 5-day therapy duration exceeds the current recommendations.
The drug is commonly used to enhance comfort among outpatients with uncomplicated UTIs. However, phenazopyridine is not routinely included in treatment protocols for complicated UTIs or inpatient scenarios addressing acute pyelonephritis in patients with risk factors for heightened infection severity.
Diagnostic use
A study indicates that the oral administration of phenazopyridine with dextrose instillation can effectively facilitate the visualization of ureteral patency during intraoperative cystoscopy.[13] Administering a single 200 mg dose of phenazopyridine to the patient on the evening before the surgical procedure aids in identifying the urethral orifice.[14] However, phenazopyridine is not valuable as a diagnostic tool for detecting incontinence or premature membrane rupture.[15][16]
Cystoscopy use
In a randomized controlled trial, 97 patients undergoing cystoscopy received either 200 mg of phenazopyridine along with lidocaine gel or lidocaine gel alone. The medications were administered in 3 doses, starting with a dose administered 20 minutes before the procedure and then every 8 hours thereafter. Participants who were administered phenazopyridine and lidocaine gel reported experiencing less discomfort and exhibited lower heart rates than those who received only lidocaine gel. The authors concluded that phenazopyridine reduces the intensity of pain associated with both cystoscopy and the initial act of urination.[17]
Postoperative use
In a randomized controlled study involving 152 women undergoing prolapse surgery, administering a single postoperative dose of phenazopyridine at 200 mg did not demonstrate enhanced postoperative voiding compared to the control group.[18] In a retrospective cohort study involving 149 women who underwent a retropubic mid-urethral sling operation, phenazopyridine was reported to improve postoperative voiding.[19]
Radiation-induced cystitis use
Phenazopyridine has been used safely for up to 2 months as a form of supportive care in cases of radiation-induced cystitis.[20]
Specific Patient Populations
Renal impairment: For individuals with mild renal impairment, with a glomerular filtration rate of more than 50 mL/min, the recommended dosing frequency is every 8 to 16 hours. Phenazopyridine should not used in patients with a glomerular filtration rate of less than 50 mL/min.[21]
Hepatic impairment: Phenazopyridine is contraindicated in cases of severe hepatitis.
Pregnancy considerations: Phenazopyridine is an FDA pregnancy category B drug known to cross the placenta.[16] Phenazopyridine should be used in pregnancy only if it is indicated.
Breastfeeding considerations: The safety of phenazopyridine is not established during breastfeeding. Therefore, phenazopyridine is not recommended during lactation due to its potential to cause methemoglobinemia and hemolytic anemia, especially in infants with glucose-6-phosphate dehydrogenase (G6PD) deficiency.[22]
Pediatric considerations: The recommended dosage for children aged 6 to 12 is 12 mg/kg/d, divided into 3 equal doses. A specialized pediatric formulation is not commercially available and requires compounding.[23] Although review articles and consensus publications addressing the treatment of pediatric UTIs exist, they do not typically reference the use of phenazopyridine.[24][25]
Older patients: It is advised to consult renal dosing guidelines for older patients.
Clinical Studies
Phenazopyridine was marketed before the implementation of regulations that mandated preclinical studies to establish a drug's safety and efficacy. Consequently, comprehensive and substantial studies are lacking, and the drug's role in UTI treatment primarily relies on clinical observations.
A critical analysis of the available studies has been published. Among these studies, 1 encompassed 118 patients, of which 64% of the participants had cystitis or pyelonephritis. All participants were administered phenazopyridine at 200 mg, 3 times daily, for 2 weeks, exceeding the recommended duration. The analysis was conducted as an open-label, single-arm study. The authors documented enhanced symptomatic responses, including alleviation of dysuria (95.3%), burning sensation (93.6%), frequency (85.6%), and nocturia (83.7%).[1]
Another investigation involved 49 subjects with acute UTIs who received phenazopyridine at a dosage of 200 mg administered 3 times daily. After 24 and 72 hours, patients underwent evaluations for nocturia, burning, and urgency symptoms. Mean symptom scores were reported as slight, with 3 or 4 symptoms after 24 hours and none, with 1 or 2 after 72 hours.[26]
Phenazopyridine was compared to flavoxate, an antispasmodic drug, in a study involving 392 participants displaying UTI symptoms, incontinence, and/or suprapubic pain. The participants were administered either phenazopyridine at a dosage of 200 mg 3 times daily or flavoxate at a dosage of 100 mg 4 times daily. The variances in clinical response did not demonstrate statistical significance. In a separate analysis of men with prostatitis, the participants were subjected to a regimen of phenazopyridine at a dosage of 200 mg 3 times daily. Only 31% of the subjects reported satisfactory relief from symptoms.[12]
Adverse Effects
Phenazopyridine has a good safety profile, with infrequent occurrences of severe adverse events. Severe and potentially life-threatening adverse effects are generally confined to instances of overdose, preexisting renal insufficiency, and surpassing the recommended dosing and duration guidelines.[27][28]
The common adverse drug reactions of phenazopyridine have been listed below.
- Central nervous system: Headache
- Dermatological: Rash, discoloration, pruritus, and ulceration [29]
- Hypersensitivity: Anaphylactoid-like reaction or hypersensitivity hepatitis
- Gastrointestinal: Nausea, vomiting, and diarrhea
- Hematological: Methemoglobinemia, sulfhemoglobinemia, and hemolytic anemia (may act as a potential hemolytic agent in G6PD deficiency) [30][31][32]
- Renal: Acute interstitial nephritis [33]
- Other effects: Discoloration of body fluids, aseptic meningitis, visual disturbances, renal or hepatic toxicity associated with overdose, jaundice, and renal calculi [4]
The drug is recognized for its potential to induce methemoglobinemia and hemolytic anemia, which is thought to occur via a metabolite, aniline. In cases of hemolytic anemia, a microscopic examination of red blood cells reveals the presence of Heinz bodies and degmacytes (bite cells).[34]
The treatment for drug-induced hemolytic anemia involves discontinuing the causative agent and contemplating the utilization of corticosteroids.[35] Interstitial nephritis may manifest following an overdose, and cases have been reported even with therapeutic doses and in individuals with normal renal function.[33] In instances of phenazopyridine-induced nephrotoxicity, the use of roentgenographic contrast media may exacerbate renal damage.[36]
Cases of hepatitis appear to be associated with dose-related hypersensitivity.[37][38] Other infrequent adverse effects, such as thrombocytopenia and allergic-like skin reactions, have also been reported.[39][40]
Contraindications
Individuals with known hypersensitivity to phenazopyridine should avoid taking the medication. Furthermore, phenazopyridine is contraindicated in severe renal insufficiency, with a glomerular filtration rate below 50 mL/min. Patients with severe hepatitis are advised not to take the drug due to contraindications.
Precautions
Patients with known G6PD deficiency are more susceptible to hemolysis and should avoid receiving phenazopyridine.[30] In addition, the drug should be administered cautiously in cases of impaired hepatic function.
Carcinogenicity
Rats subjected to phenazopyridine exhibited the development of liver and colorectal tumors, encompassing both malignant and benign growths. However, no established correlation exists between human phenazopyridine usage and carcinogenicity (15th Report on Carcinogens, National Toxicology Program, 2021).
Interactions
Phenazopyridine has no known drug-drug or drug-food interactions. Due to its dye properties, phenazopyridine is anticipated to interfere with urinalysis tests that rely on color reactions or spectrometry. Theoretically, there might be a heightened risk of methemoglobinemia when phenazopyridine is combined with a local anesthetic such as benzocaine. However, this potential risk lacks confirmation in medical literature, as the drug has been effectively employed alongside lidocaine gel.[17]
Monitoring
Because phenazopyridine is available as an OTC product, there is the possibility of incorrect drug usage. Effective patient education is of utmost importance while using the medication.[41] Healthcare providers should instruct patients that phenazopyridine is not an antibiotic but provides only symptomatic relief.[42]
Although some uncomplicated lower UTIs might resolve spontaneously and even achieve a bacteriological cure, the overall cure rate remains relatively low. Research findings have established that appropriate antibiotic treatment leads to quicker symptom resolution and a higher likelihood of bacteriological cure than a placebo.[43][44] In cases where an antibiotic is prescribed, phenazopyridine enhances comfort while the antibiotic works to eliminate the causative bacteria.
In 2 surveys conducted in Los Angeles County, 71% of OTC phenazopyridine consumers were unaware that a bladder infection or another type of UTI caused their symptoms. Furthermore, 38% reported purchasing the drug as a substitute for medical care.[45][46]
Due to its dye properties, phenazopyridine causes a noticeable change in urine color, turning it reddish-orange (see Image. Urine Colored With Phenazopyridine). Patients should receive counseling about this effect to address concerns proactively. They should be informed that the alteration in their urine color results from the drug's color and does not indicate bleeding. Spilled urine can potentially cause staining, particularly on clothing. Extended use beyond appropriate limits may lead to a yellowing of the skin and the sclera of the eyes. The drug is also reported to affect the color of tears and ejaculate, potentially leading to the staining of contact lenses. Patients should also promptly report any urinary tract symptoms that do not improve or worsen.[23][41]
Toxicity
The occurrence of phenazopyridine-induced methemoglobinemia is rare, with fewer than 50 cases being documented since 1951. The National Poison Data System Study conducted in 2020 investigated patients from 2007 to 2017 to identify the substances frequently associated with methemoglobinemia. The study underscores that phenazopyridine is linked to a notable number of reported cases of methemoglobinemia.[47]
Methemoglobinemia can be managed using standard treatments, including methylene blue, the preferred antidote. This medication is administered intravenously as a 1% solution at a dose of 1 to 2 mg/kg as required. Methylene blue should not be administered to patients with known or suspected G6PD deficiency and those taking antidepressants, as it can elevate the risk of serotonin syndrome.[48]
In such scenarios, the recommended alternative antidote is intravenous high-dose ascorbic acid. Research has elucidated the reasons why phenazopyridine and other drugs, such as dapsone and chloroquine, more commonly induce methemoglobinemia compared to other medications. Oxidative Heinz body hemolytic anemia may also be triggered, and chronic overdosage incidents can manifest with bite cells, also known as degmacytes.
In cases of drug overdose, patients can experience renal toxicity, occasional renal failure, and hepatic impairment.[49] Although the mechanism behind renal toxicity remains unclear, there is a possibility that a metabolite known as triaminopyridine might damage the distal renal tubules.[11] Life-threatening adverse effects, including hemolytic anemia and renal failure, have been associated with inappropriate OTC phenazopyridine use and intentional drug overdose.[50][51]
Enhancing Healthcare Team Outcomes
Due to its availability as an OTC product, there exists the potential for inappropriate use of phenazopyridine. Therefore, gathering information on both OTC and prescription drugs is imperative when conducting medication reviews.[51]
The interprofessional healthcare team should collaborate to deliver comprehensive patient education. Opting for treatment with an appropriate antibiotic leads to significantly faster symptom relief and the elimination of the infection Clinicians may consider prescribing phenazopyridine to alleviate the sensations of burning, urgency, frequency, and pain linked to lower UTIs, potentially for a brief duration of 2 days until the antibiotic effectively eliminates the causative bacteria Continued use beyond 2 days could impede the timely diagnosis and initiation of appropriate treatment. Hence, patients must understand that phenazopyridine does not possess any anti-infective properties.
Healthcare providers should verify the accuracy of the dosage before administering it to patients and counsel them regarding the extended treatment duration, which heightens the risk of severe adverse effects. Patients should also be informed about urine color changes to prevent unnecessary alarm when they observe this alteration.
Pharmacists are critical in educating patients that phenazopyridine solely offers symptomatic relief and, therefore, should not serve as a substitute for seeking medical attention. Instead, patients should collaborate with their medical providers, sharing any observations or concerns about their medication regimen. Promoting open communication and fostering collaborative efforts among all interprofessional healthcare team members enhances patient safety and optimize treatment outcomes when utilizing phenazopyridine.