Continuing Education Activity
Transrectal prostate ultrasound is a key tool for diagnosing many prostatic diseases, such as prostate cancer, prostatitis, and benign prostatic hyperplasia. Besides traditional grayscale imaging, multiple additional technologies may aid in the diagnosis. This activity outlines the transrectal ultrasonographic evaluation of the prostate and highlights the interprofessional team's role in improving care for patients with prostatic disease.
Objectives:
- Review the indications for a transrectal prostate ultrasound.
- Describe the typical imaging findings of prostate cancer, prostatitis, and benign prostatic hyperplasia.
- Identify the most common anatomical zones for prostate cancer and benign prostatic hyperplasia.
- Explain the importance of the added technologies for diagnosing prostatic disease with transrectal prostate ultrasound.
Introduction
Transrectal ultrasonography (TRUS) is a staple of prostate evaluation. The technique was first described in 1968 by Watanabe and colleagues. However, it did not obtain widespread use until a couple of decades later. It is now commonly utilized for imaging any prostatic pathology, especially prostate cancer, prostatitis, and benign prostatic hyperplasia (BPH).[1]
Prostate cancer is the most common new cancer diagnosis in men, and the incidence is only expected to increase as the population ages. The widespread routine monitoring of prostate-specific antigen (PSA) has increased the overall diagnosis of cancer. However, these are mostly low-grade neoplasms. The most widely used diagnostic tool to evaluate prostate cancer is transrectal ultrasound, and it also serves as a guide for biopsy.
Prostatitis is a fairly common disease that was classically diagnosed on physical exam and laboratory findings alone. Still, imaging has played an increasing role with the more recent advancements of TRUS. BPH is extremely prevalent, affecting 20% of men aged 30 to 79, with 80% of men being affected by age 70. Imaging is not required for diagnosis. However, the gland changes are easily recognized, and it is recommended if any surgical intervention is planned.[2][3][4][5][6]
Anatomy and Physiology
The prostate is a genitourinary organ in men located between the bladder and urogenital diaphragm. It is shaped like a walnut and measures on average 3.75 to 4.0 x 2.5-3.0 x 3.1 to 3.8 cm (width x length x height) with a volume of 20-25 cm3. The prostatic base is located adjacent to the bladder with its apex at the urogenital diaphragm. The seminal vesicles join with the vas deferens at the prostate's posterior aspect to form the paired ejaculatory ducts, which traverse the gland and empty into the prostatic urethra.[7][8]
The prostate is most commonly classified into anatomic zones: the peripheral, central, transitional, periurethral zones, and the anterior fibromuscular stroma, containing non-glandular tissue. The peripheral zone accounts for 75% of the prostate's mass and is where most prostate cancers arise. Prostate cancer is unique in that it is typically multifocal, whereas other malignancies tend to be unifocal unless they have metastasized. The central zone contains glandular tissue and is where the ejaculatory ducts travel to empty into the urethra. The transitional zone surrounds the urethra and is the smallest component. However, it is the primary site of benign prostatic hyperplasia. These different zones are not readily differentiated on imaging. Therefore on the transrectal ultrasound, the gland is divided into an inner (periurethral and transitional zones) and outer (central, peripheral, anterior stromal zones) gland, separated by the surgical capsule.[1]
The prostate is supplied by three branches of the internal iliac arteries: internal pudendal, inferior vesicle, and middle rectal arteries. It receives venous blood from the dorsal vein of the penis and drains into the iliac veins. There is also a communication to the internal vertebral venous plexus (Batson plexus), which accounts for the metastatic spread of prostate cancer to the vertebral bodies. The lymphatic drainage is primarily to the internal iliac and sacral lymph nodes. It receives parasympathetic supply from pelvic splanchnic nerves (S2-4) and sympathetic supply from the inferior hypogastric plexuses. The neurovascular bundle travels posterolateral to the prostate.
The prostate has multiple physiologic functions. The glandular tissue produces the glycoprotein prostate-specific antigen (PSA), which liquefies semen, multiple prostaglandins (A, E, and F), spermidine, and spermine. The gland also is the site of the conversion of testosterone into dihydrotestosterone with the enzyme 5α reductase.[7]
Indications
There are many indications for transrectal prostate ultrasound, including:
- Abnormal PSA (>4 ng/mL)
- Abnormality palpated on digital rectal examination
- Suspected infection (prostatitis or prostatic abscess)
- To evaluate for BPH due to lower urinary tract symptoms (LUTS) or abnormality seen on transabdominal imaging
- To aid and guide transrectal biopsy[9]
Contraindications
There are no prostatic diseases that are contraindications to transrectal ultrasound. However, unrelated conditions may preclude the insertion of the probe, such as rectal mass or other anorectal diseases (i.e., hemorrhoids, local dermatologic condition, stricture). These are assessed on the digital rectal exam performed before probe insertion.[8]
Equipment
For this technique, endorectal linear or convex transducers with high frequencies are used (7.5 to 10 MHz are acceptable, however, the higher, the better). These may be single or biplane transducers. However, biplane transducers are preferred as probes do not have to be exchanged to view both transverse and sagittal planes necessary for a complete evaluation, decreasing the patient's overall discomfort. Images are obtained using B-mode.
There are supplemental tools that may be utilized for evaluation that vary in availability based on the institution:
- Color and power Doppler are universally available and assess vascularity best used when there is a concern for inflammatory conditions.
- Elastography is an emerging technology that helps to identify differences in tissue stiffness, such as in prostate cancer.
- 3D ultrasound gives a more detailed view of the prostate and allows evaluation in the coronal plane and spatial relationships of the prostatic capsule and adjacent structures.
- Contrast-enhanced ultrasound uses microbubble contrast agents to better demonstrate neovascularity and increased vascular density associated with malignancy.
If the ultrasound is being done to guide biopsy, the preferred probe is a biplane. It must contain an internal bioptic channel or attached biopsy needle guide, which corresponds to the biopsy track demonstrated on the screen.[9][10]
Preparation
Depending on the institution, a suppository or enema may be ordered before the ultrasound to ensure that the rectum is empty. However, this is controversial.[11]
Before the ultrasound probe insertion, a digital rectal exam must be performed to assess for any possible contraindications as listed above.[9]
Technique or Treatment
The patient is placed in the lateral decubitus position (most commonly left lateral) with their knees flexed to their chest. The lithotomy position was previously the standard. However, lateral decubitus is better tolerated by the patient. The transducer is covered and well lubricated. After the rectal exam, the transducer is inserted with the ultrasound directed anteriorly in respect to the patient. The least amount of pressure possible on the prostate gland should be applied to avoid altering the contour of the gland.
Scanning is typically first done in the transverse plane, from the base towards the apex. In this plane, the symmetry and shape are assessed, as well as the width of the gland and the associated seminal vesicles. Once the gland is evaluated in the transverse axis, either the probe is removed (if it is a single planar probe) or the axis is switched (if it is biplanar). The prostate is then evaluated in the sagittal plane, assessing the height and length as well as the seminal vesicles and prostatic urethra.
Overall echogenicity (focal and diffuse) and changes in echogenicity are primary considerations in the evaluation. To obtain the best imaging, magnification and gain should be adjusted such that the prostate is filling the screen and the peripheral zone is a medium gray. Additionally, if clinically warranted, the bladder may be assessed before and after voiding to evaluate for postvoid residual volume.[12][9][13]
Throughout the exam, multiple additional features may be used to further assess the prostate, as described above:
- Color and power Doppler may be applied to any grayscale image.
- There are two different elastography techniques:
- Compression elastography measures the amount of displacement after repetitive compression and decompression while scanning the prostate.
- Shear-wave elastography delivers shear waves to the tissue and measures the velocity of those waves. Velocity is then associated with how stiff the tissue is, i.e., waves move faster through stiffer tissue. Images are obtained by avoiding placing pressure on the prostate.
- Microbubble contrast agents are injected and, because of their small size (<1µm), pass through the pulmonary vasculature and can enhance end organs. Enhancement after bolus injection typically lasts only 1 to 2 minutes; slow intravenous infusion can instead be used to extend this enhancement. This is classically imaged with either Doppler or grayscale harmonic. However, newer techniques may also allow for increased penetration of the contrast into the organ (intermittent harmonic imaging or flash-replenishment technique).
- 3D images are obtained by slowly sliding the probe over the entire prostate from apex to base. The data is saved as voxels and can be viewed in any plane, providing a better assessment of spatial relationships.[10][9]
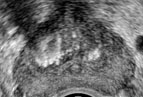
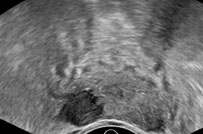
Prostate cancer typically appears as a hypoechoic lesion in the outer gland (peripheral zone) and is often multifocal. However, the appearance is variable and may also appear isoechoic (only 1% are hyperechoic), which makes identifying these lesions more difficult, and therefore additional techniques are necessary for diagnosis. Increased color or power Doppler flow may be seen. However, this is not as sensitive and will likely only be seen in higher-grade cancers. Contrast-enhanced ultrasound will demonstrate increased flow due to increased microvessel density.
The areas of cancer will have decreased elasticity due to increased cell density. This helps confirm the diagnosis and may also guide biopsy. Tumor extension can be assessed with grayscale imaging and may demonstrate bulges and irregularity of the prostatic contour as well as disrupted periprostatic fat. A hyperechoic seminal vesicle, asymmetric enlargement, or anterior displacement (altered prostate-seminal vesicle angle) all suggest the involvement of the seminal vesicle. The presence of extracapsular extension may also be assessed with 3D ultrasonography in place of cross-sectional imaging to better demonstrate spatial relationships. One additional important relationship to evaluate is the relationship of cancer to the neurovascular bundle, which travels along the posterolateral aspect. If cancer spares this region, it allows the possibility of nerve-sparing prostatectomy.[1][8]
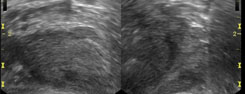
Prostatitis may make the ovoid prostate appear rounded and decrease the echogenicity due to edema. This causes blurring of the distinction between the inner and outer gland. It may also create a heterogeneous appearance of the gland with small hypoechoic areas (possible early abscesses). The classic sign is a hypoechoic halo in the periurethral zone, which is fairly specific for prostatitis. However, this may be confused with the normal hypoechoic periprostatic sphincter. There will typically be increased vascularity seen on color or power Doppler. Chronic or recurrent infections may lead to dystrophic calcifications in the apical peripheral zone or fibrosis. An abscess is a late complication of prostatitis. This will appear the same as in other areas of the body and is typically found within the transitional zone.[4]
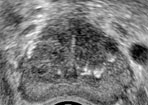
Benign prostatic hyperplasia will appear as multiple hypoechoic nodular areas within the transitional zone. It may progress from fairly homogenous to heterogeneous echogenicity. As the transitional zone enlarges, it may start to compress the peripheral and central zones, causing them to become echogenic. As they flatten, they are known as the “surgical capsule,” which is the line of demarcation between the hyperplastic and normal peripheral prostatic tissue. The overall volume of the prostate is crucial to evaluate when concerned with BPH. The most widely used formula is for the volume of an ellipsoid: height (H) x width (W) x length (L) x π/6. An enlarged prostate is diagnosed when the volume is greater than 25 cm3.[6][11]
Complications
There are no known complications from transrectal prostate ultrasonography. However, there are minor complications if the ultrasound was performed in conjunction with a prostate biopsy, such as bleeding and infection.[11]
Clinical Significance
Transrectal ultrasound of the prostate is a crucial tool in the evaluation of prostate pathology. Understanding of the techniques used allows tests to be ordered for the correct scenarios and possibly in situations they may not have been ordered in the past. Advancing techniques also allow for better diagnosis and alleviate the need for cross-sectional imaging and, therefore, radiation. Given the increasing burden of prostate cancer, infection, and BPH on the healthcare system, inexpensive diagnostic tools, provide a prompt diagnosis, and are easy to perform will become even more important. That is what TRUS represents.
Enhancing Healthcare Team Outcomes
Relationships between the sonographer, radiologist, and ordering physician (urologist or primary care clinician) are crucial to optimize patient-centered care. As with any diagnostic data, interpretation and subsequent action will require the efforts of an interprofessional healthcare team, including clinicians, mid-level practitioners, nurses, radiologists, and ultrasound techs, all working collaboratively and sharing information openly to drive optimal patient outcomes. [Level 5]
A good working relationship between the radiologist and ordering physician is key to having an open dialogue about the indications for and the extent of diagnostic capabilities of TRUS to ensure that testing is ordered correctly. As new technologies develop or are made available at certain institutions, communication should increase accordingly. This is especially important as machine capabilities vary between institutions, and the use of new technologies can improve patient care by providing increased and more accurate information.
The relationship between the radiologist and sonographer is equally as important. As the radiologist is limited by the sonographer's images, a common understanding of the technique is crucial to avoid any missed or inaccurate diagnoses.