[1]
Expert Panel on Women’s Imaging.,Wall DJ,Reinhold C,Akin EA,Ascher SM,Brook OR,Dassel M,Henrichsen TL,Learman LA,Maturen KE,Patlas MN,Robbins JB,Sadowski EA,Saphier C,Uyeda JW,Glanc P, ACR Appropriateness Criteria® Female Infertility. Journal of the American College of Radiology : JACR. 2020 May;
[PubMed PMID: 32370955]
[2]
Baerwald AR,Pierson RA, Endometrial development in association with ovarian follicular waves during the menstrual cycle. Ultrasound in obstetrics
[PubMed PMID: 15343603]
[3]
Raine-Fenning NJ,Campbell BK,Clewes JS,Kendall NR,Johnson IR, Defining endometrial growth during the menstrual cycle with three-dimensional ultrasound. BJOG : an international journal of obstetrics and gynaecology. 2004 Sep;
[PubMed PMID: 15327609]
[4]
Bakos O,Lundkvist O,Wide L,Bergh T, Ultrasonographical and hormonal description of the normal ovulatory menstrual cycle. Acta obstetricia et gynecologica Scandinavica. 1994 Nov;
[PubMed PMID: 7817731]
[5]
Hershko-Klement A,Tepper R, Ultrasound in assisted reproduction: a call to fill the endometrial gap. Fertility and sterility. 2016 Jun;
[PubMed PMID: 27140291]
[6]
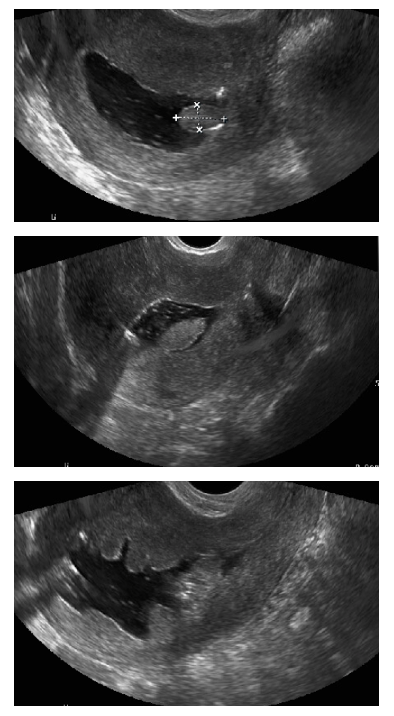
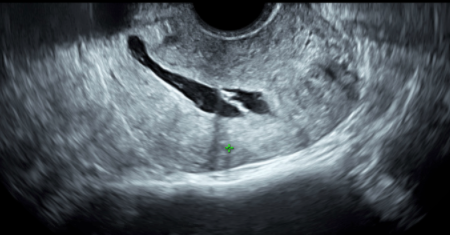
Leone FP,Timmerman D,Bourne T,Valentin L,Epstein E,Goldstein SR,Marret H,Parsons AK,Gull B,Istre O,Sepulveda W,Ferrazzi E,Van den Bosch T, Terms, definitions and measurements to describe the sonographic features of the endometrium and intrauterine lesions: a consensus opinion from the International Endometrial Tumor Analysis (IETA) group. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2010 Jan
[PubMed PMID: 20014360]
Level 3 (low-level) evidence
[7]
Chien LW,Au HK,Chen PL,Xiao J,Tzeng CR, Assessment of uterine receptivity by the endometrial-subendometrial blood flow distribution pattern in women undergoing in vitro fertilization-embryo transfer. Fertility and sterility. 2002 Aug;
[PubMed PMID: 12137858]
[8]
Faddy MJ,Gosden RG,Gougeon A,Richardson SJ,Nelson JF, Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Human reproduction (Oxford, England). 1992 Nov;
[PubMed PMID: 1291557]
[9]
Faddy MJ,Gosden RG, A model conforming the decline in follicle numbers to the age of menopause in women. Human reproduction (Oxford, England). 1996 Jul;
[PubMed PMID: 8671489]
[10]
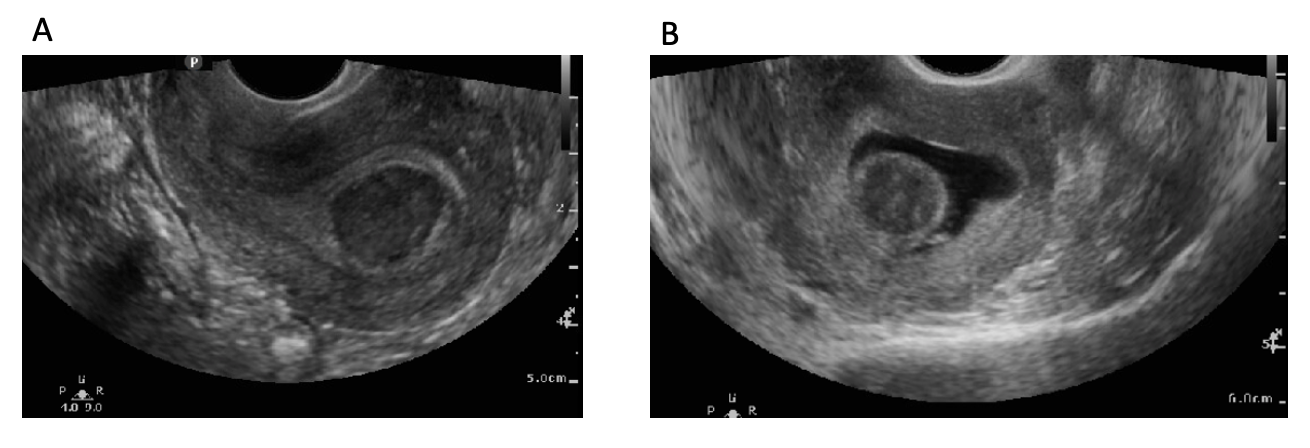
Coelho Neto MA,Ludwin A,Borrell A,Benacerraf B,Dewailly D,da Silva Costa F,Condous G,Alcazar JL,Jokubkiene L,Guerriero S,Van den Bosch T,Martins WP, Counting ovarian antral follicles by ultrasound: a practical guide. Ultrasound in obstetrics
[PubMed PMID: 29080259]
[11]
Klenov VE,VAN Voorhis BJ, Ultrasound in Infertility Treatments. Clinical obstetrics and gynecology. 2017 Mar;
[PubMed PMID: 28059846]
[12]
Mikolajczyk RT,Stanford JB,Ecochard R, Multilevel model to assess sources of variation in follicular growth close to the time of ovulation in women with normal fertility: a multicenter observational study. Reproductive biology and endocrinology : RB
[PubMed PMID: 19077200]
Level 2 (mid-level) evidence
[13]
Hancock KL,Pereira N,Christos PJ,Petrini AC,Hughes J,Chung PH,Rosenwaks Z, Optimal lead follicle size for human chorionic gonadotropin trigger in clomiphene citrate and intrauterine insemination cycles: an analysis of 1,676 treatment cycles. Fertility and sterility. 2021 Apr;
[PubMed PMID: 33272641]
[14]
AIUM Practice Parameter for the Performance of a Focused Ultrasound Examination in Reproductive Endocrinology and Female Infertility. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2019 Mar;
[PubMed PMID: 30758891]
[15]
Hudelist G,Fritzer N,Staettner S,Tammaa A,Tinelli A,Sparic R,Keckstein J, Uterine sliding sign: a simple sonographic predictor for presence of deep infiltrating endometriosis of the rectum. Ultrasound in obstetrics
[PubMed PMID: 23400893]
[16]
Campbell S, Ultrasound Evaluation in Female Infertility: Part 1, the Ovary and the Follicle. Obstetrics and gynecology clinics of North America. 2019 Dec;
[PubMed PMID: 31677749]
[18]
Jayaprakasan K,Chan Y,Islam R,Haoula Z,Hopkisson J,Coomarasamy A,Raine-Fenning N, Prediction of in�vitro fertilization outcome at different antral follicle count thresholds in a prospective cohort of 1,012 women. Fertility and sterility. 2012 Sep;
[PubMed PMID: 22749225]
[19]
Depmann M,Broer SL,van der Schouw YT,Tehrani FR,Eijkemans MJ,Mol BW,Broekmans FJ, Can we predict age at natural menopause using ovarian reserve tests or mother's age at menopause? A systematic literature review. Menopause (New York, N.Y.). 2016 Feb;
[PubMed PMID: 26372034]
Level 1 (high-level) evidence
[20]
Wellons MF,Bates GW,Schreiner PJ,Siscovick DS,Sternfeld B,Lewis CE, Antral follicle count predicts natural menopause in a population-based sample: the Coronary Artery Risk Development in Young Adults Women's Study. Menopause (New York, N.Y.). 2013 Aug;
[PubMed PMID: 23422869]
[21]
Wittmaack FM,Kreger DO,Blasco L,Tureck RW,Mastroianni L Jr,Lessey BA, Effect of follicular size on oocyte retrieval, fertilization, cleavage, and embryo quality in in vitro fertilization cycles: a 6-year data collection. Fertility and sterility. 1994 Dec;
[PubMed PMID: 7957985]
Level 2 (mid-level) evidence
[22]
Evans MB,Stentz NC,Richter KS,Schexnayder B,Connell M,Healy MW,Devine K,Widra E,Stillman R,DeCherney AH,Hill MJ, Mature Follicle Count and Multiple Gestation Risk Based on Patient Age in Intrauterine Insemination Cycles With Ovarian Stimulation. Obstetrics and gynecology. 2020 May;
[PubMed PMID: 32282611]
[23]
Teede HJ,Misso ML,Costello MF,Dokras A,Laven J,Moran L,Piltonen T,Norman RJ,International PCOS Network., Recommendations from the international evidence-based guideline for the assessment and management of polycystic ovary syndrome. Fertility and sterility. 2018 Aug;
[PubMed PMID: 30033227]
Level 1 (high-level) evidence
[24]
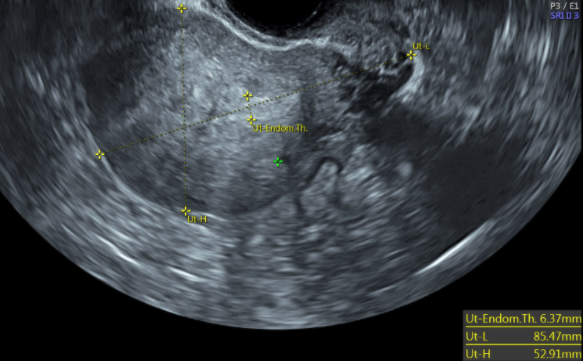
Zhao J,Zhang Q,Li Y, The effect of endometrial thickness and pattern measured by ultrasonography on pregnancy outcomes during IVF-ET cycles. Reproductive biology and endocrinology : RB
[PubMed PMID: 23190428]
[25]
Fleischer AC,Pittaway DE,Beard LA,Thieme GA,Bundy AL,James AE Jr,Wentz AC, Sonographic depiction of endometrial changes occurring with ovulation induction. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 1984 Aug;
[PubMed PMID: 6434749]
[26]
Wu Y,Gao X,Lu X,Xi J,Jiang S,Sun Y,Xi X, Endometrial thickness affects the outcome of in vitro fertilization and embryo transfer in normal responders after GnRH antagonist administration. Reproductive biology and endocrinology : RB
[PubMed PMID: 25296555]
[27]
Traub ML,Van Arsdale A,Pal L,Jindal S,Santoro N, Endometrial thickness, Caucasian ethnicity, and age predict clinical pregnancy following fresh blastocyst embryo transfer: a retrospective cohort. Reproductive biology and endocrinology : RB
[PubMed PMID: 19386129]
Level 2 (mid-level) evidence
[28]
Zhang T,Li Z,Ren X,Huang B,Zhu G,Yang W,Jin L, Endometrial thickness as a predictor of the reproductive outcomes in fresh and frozen embryo transfer cycles: A retrospective cohort study of 1512 IVF cycles with morphologically good-quality blastocyst. Medicine. 2018 Jan;
[PubMed PMID: 29369190]
Level 2 (mid-level) evidence
[29]
Wang L,Qiao J,Li R,Zhen X,Liu Z, Role of endometrial blood flow assessment with color Doppler energy in predicting pregnancy outcome of IVF-ET cycles. Reproductive biology and endocrinology : RB&E. 2010 Oct 18
[PubMed PMID: 20955593]
Level 2 (mid-level) evidence
[30]
Zaidi J,Campbell S,Pittrof R,Tan SL, Endometrial thickness, morphology, vascular penetration and velocimetry in predicting implantation in an in vitro fertilization program. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 1995 Sep
[PubMed PMID: 8521069]
[31]
Richter KS,Bugge KR,Bromer JG,Levy MJ, Relationship between endometrial thickness and embryo implantation, based on 1,294 cycles of in vitro fertilization with transfer of two blastocyst-stage embryos. Fertility and sterility. 2007 Jan
[PubMed PMID: 17081537]
[32]
Munro MG, Uterine polyps, adenomyosis, leiomyomas, and endometrial receptivity. Fertility and sterility. 2019 Apr
[PubMed PMID: 30929720]
[33]
Soares SR,Barbosa dos Reis MM,Camargos AF, Diagnostic accuracy of sonohysterography, transvaginal sonography, and hysterosalpingography in patients with uterine cavity diseases. Fertility and sterility. 2000 Feb
[PubMed PMID: 10685551]
[34]
Farquhar C,Ekeroma A,Furness S,Arroll B, A systematic review of transvaginal ultrasonography, sonohysterography and hysteroscopy for the investigation of abnormal uterine bleeding in premenopausal women. Acta obstetricia et gynecologica Scandinavica. 2003 Jun
[PubMed PMID: 12780419]
Level 1 (high-level) evidence
[35]
Hinckley MD,Milki AA, 1000 office-based hysteroscopies prior to in vitro fertilization: feasibility and findings. JSLS : Journal of the Society of Laparoendoscopic Surgeons. 2004 Apr-Jun
[PubMed PMID: 15119651]
Level 2 (mid-level) evidence
[36]
American Association of Gynecologic Laparoscopists., AAGL practice report: practice guidelines for the diagnosis and management of endometrial polyps. Journal of minimally invasive gynecology. 2012 Jan-Feb
[PubMed PMID: 22196255]
Level 1 (high-level) evidence
[37]
Amin TN,Saridogan E,Jurkovic D, Ultrasound and intrauterine adhesions: a novel structured approach to diagnosis and management. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2015 Aug
[PubMed PMID: 26094824]
[39]
Casini ML,Rossi F,Agostini R,Unfer V, Effects of the position of fibroids on fertility. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology. 2006 Feb
[PubMed PMID: 16603437]
Level 2 (mid-level) evidence
[40]
Somigliana E,Vercellini P,Daguati R,Pasin R,De Giorgi O,Crosignani PG, Fibroids and female reproduction: a critical analysis of the evidence. Human reproduction update. 2007 Sep-Oct
[PubMed PMID: 17584819]
[41]
Sunkara SK,Khairy M,El-Toukhy T,Khalaf Y,Coomarasamy A, The effect of intramural fibroids without uterine cavity involvement on the outcome of IVF treatment: a systematic review and meta-analysis. Human reproduction (Oxford, England). 2010 Feb
[PubMed PMID: 19910322]
Level 1 (high-level) evidence
[42]
Practice Committee of the American Society for Reproductive Medicine. Electronic address: ASRM@asrm.org.,Practice Committee of the American Society for Reproductive Medicine., Removal of myomas in asymptomatic patients to improve fertility and/or reduce miscarriage rate: a guideline. Fertility and sterility. 2017 Sep
[PubMed PMID: 28865538]
[43]
Sakhel K,Abuhamad A, Sonography of adenomyosis. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2012 May
[PubMed PMID: 22535729]
[44]
Meredith SM, Sanchez-Ramos L, Kaunitz AM. Diagnostic accuracy of transvaginal sonography for the diagnosis of adenomyosis: systematic review and metaanalysis. American journal of obstetrics and gynecology. 2009 Jul:201(1):107.e1-6. doi: 10.1016/j.ajog.2009.03.021. Epub 2009 Apr 26
[PubMed PMID: 19398089]
Level 1 (high-level) evidence
[45]
Bazot M,Cortez A,Darai E,Rouger J,Chopier J,Antoine JM,Uzan S, Ultrasonography compared with magnetic resonance imaging for the diagnosis of adenomyosis: correlation with histopathology. Human reproduction (Oxford, England). 2001 Nov;
[PubMed PMID: 11679533]
[46]
Chan YY,Jayaprakasan K,Tan A,Thornton JG,Coomarasamy A,Raine-Fenning NJ, Reproductive outcomes in women with congenital uterine anomalies: a systematic review. Ultrasound in obstetrics & gynecology : the official journal of the International Society of Ultrasound in Obstetrics and Gynecology. 2011 Oct
[PubMed PMID: 21830244]
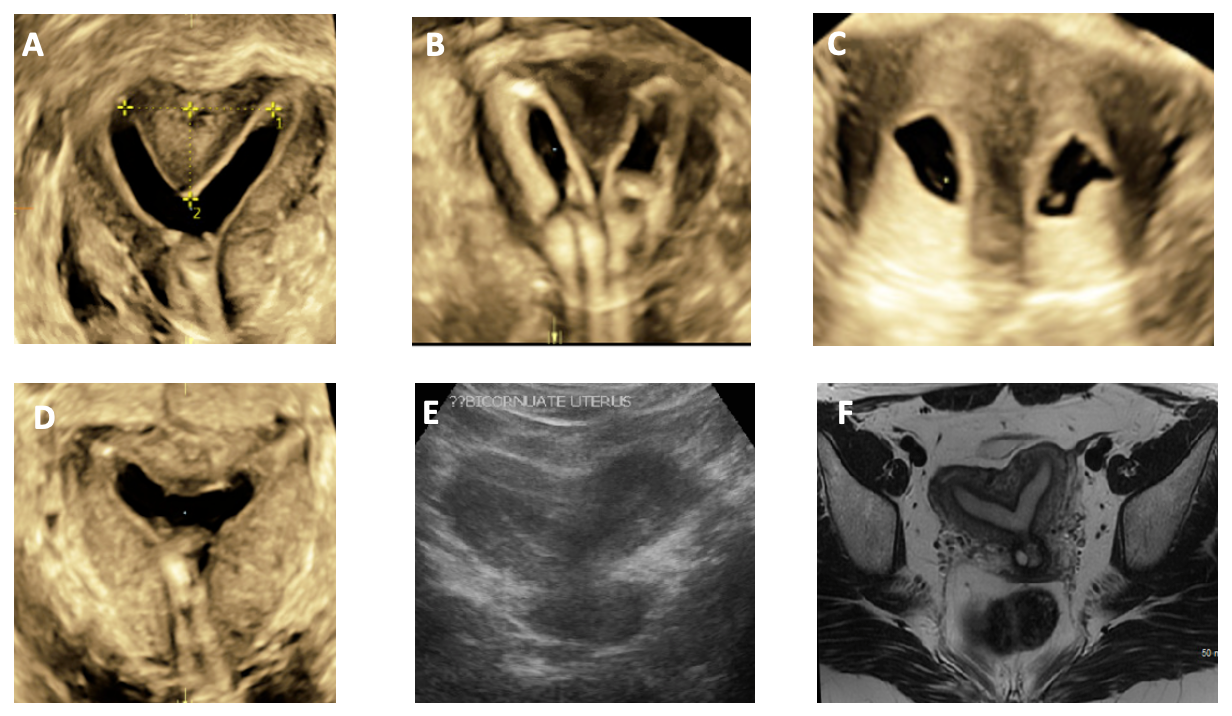
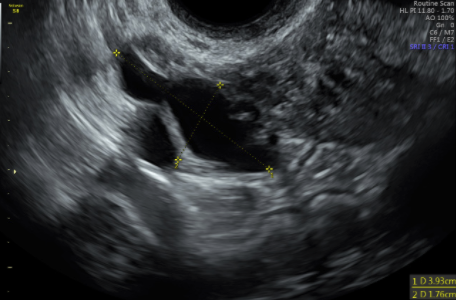
Level 1 (high-level) evidence
[47]
Carbonnel M,Pirtea P,de Ziegler D,Ayoubi JM, Uterine factors in recurrent pregnancy losses. Fertility and sterility. 2021 Mar
[PubMed PMID: 33712099]
[48]
Deutch TD, Abuhamad AZ. The role of 3-dimensional ultrasonography and magnetic resonance imaging in the diagnosis of müllerian duct anomalies: a review of the literature. Journal of ultrasound in medicine : official journal of the American Institute of Ultrasound in Medicine. 2008 Mar:27(3):413-23
[PubMed PMID: 18314520]
[49]
de Wit W,Gowrising CJ,Kuik DJ,Lens JW,Schats R, Only hydrosalpinges visible on ultrasound are associated with reduced implantation and pregnancy rates after in-vitro fertilization. Human reproduction (Oxford, England). 1998 Jun
[PubMed PMID: 9688415]
[50]
Cohen MA,Lindheim SR,Sauer MV, Hydrosalpinges adversely affect implantation in donor oocyte cycles. Human reproduction (Oxford, England). 1999 Apr;
[PubMed PMID: 10221245]
[51]
Practice Committee of American Society for Reproductive Medicine in collaboration with Society of Reproductive Surgeons., Salpingectomy for hydrosalpinx prior to in vitro fertilization. Fertility and sterility. 2008 Nov
[PubMed PMID: 19007649]
[52]
Groszmann YS,Benacerraf BR, Complete evaluation of anatomy and morphology of the infertile patient in a single visit; the modern infertility pelvic ultrasound examination. Fertility and sterility. 2016 Jun
[PubMed PMID: 27054310]
[53]
Luciano DE,Exacoustos C,Luciano AA, Contrast ultrasonography for tubal patency. Journal of minimally invasive gynecology. 2014 Nov-Dec
[PubMed PMID: 24910933]
[54]
Sallam HN,Sadek SS, Ultrasound-guided embryo transfer: a meta-analysis of randomized controlled trials. Fertility and sterility. 2003 Oct;
[PubMed PMID: 14556831]
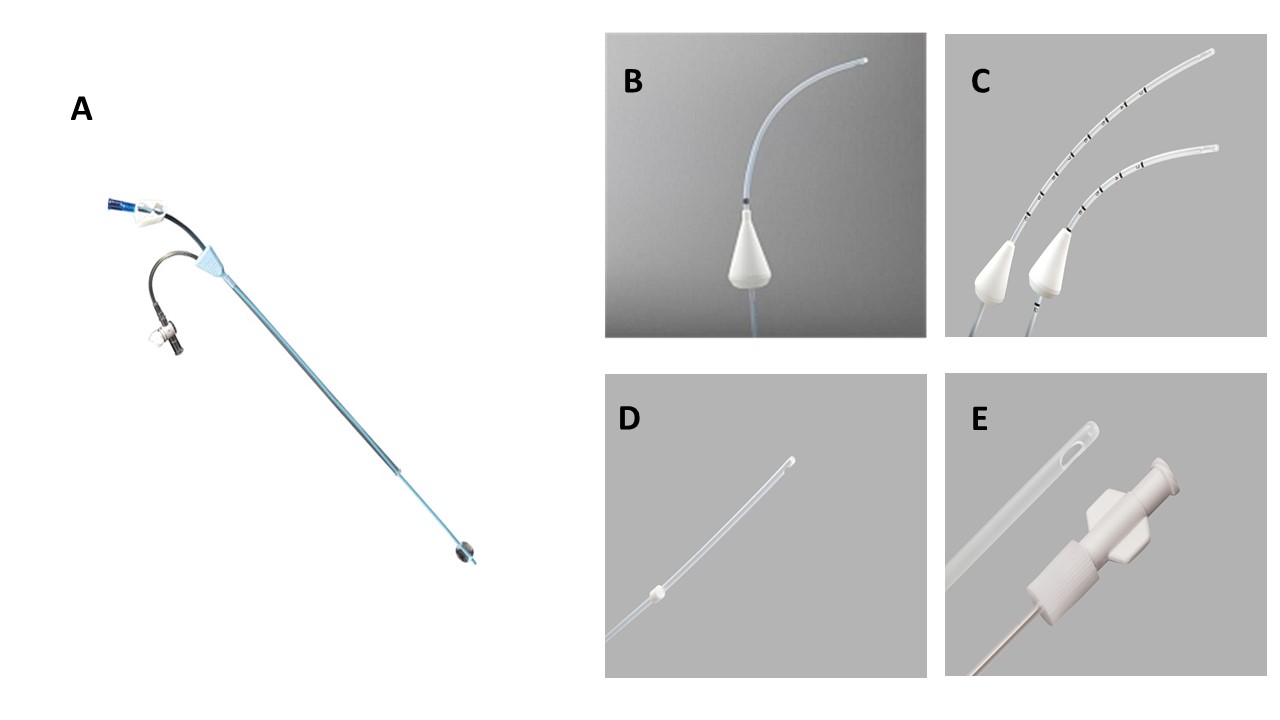
Level 1 (high-level) evidence
[55]
Cozzolino M,Vitagliano A,Di Giovanni MV,Lagan� AS,Vitale SG,Blaganje M,Drusany Stari? K,Borut K,Patrelli TS,Noventa M, Ultrasound-guided embryo transfer: summary of the evidence and new perspectives. A systematic review and meta-analysis. Reproductive biomedicine online. 2018 May;
[PubMed PMID: 29576332]
Level 3 (low-level) evidence