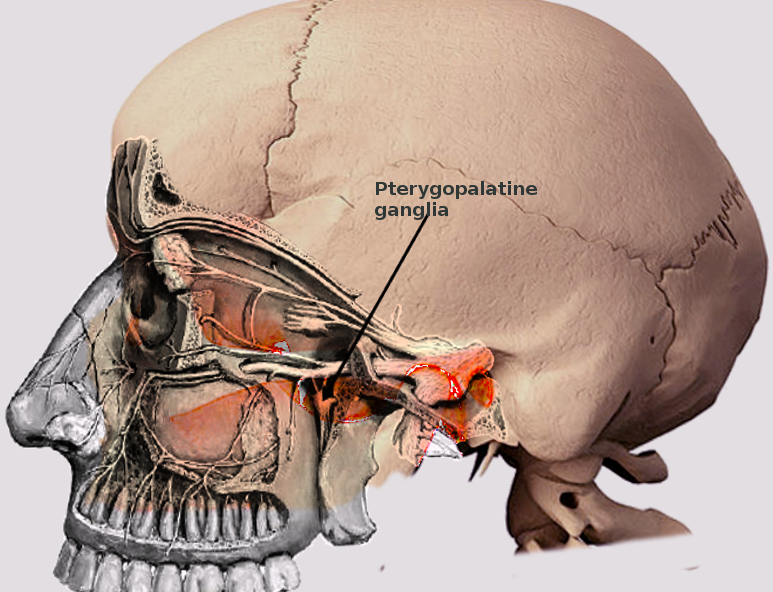
[1]
Robbins MS, Robertson CE, Kaplan E, Ailani J, Charleston L 4th, Kuruvilla D, Blumenfeld A, Berliner R, Rosen NL, Duarte R, Vidwan J, Halker RB, Gill N, Ashkenazi A. The Sphenopalatine Ganglion: Anatomy, Pathophysiology, and Therapeutic Targeting in Headache. Headache. 2016 Feb:56(2):240-58. doi: 10.1111/head.12729. Epub 2015 Nov 30
[PubMed PMID: 26615983]
[2]
Piagkou M, Demesticha T, Troupis T, Vlasis K, Skandalakis P, Makri A, Mazarakis A, Lappas D, Piagkos G, Johnson EO. The pterygopalatine ganglion and its role in various pain syndromes: from anatomy to clinical practice. Pain practice : the official journal of World Institute of Pain. 2012 Jun:12(5):399-412. doi: 10.1111/j.1533-2500.2011.00507.x. Epub 2011 Sep 29
[PubMed PMID: 21956040]
[3]
Tolba R, Weiss AL, Denis DJ. Sphenopalatine Ganglion Block and Radiofrequency Ablation: Technical Notes and Efficacy. The Ochsner journal. 2019 Spring:19(1):32-37. doi: 10.31486/toj.18.0163. Epub
[PubMed PMID: 30983899]
[4]
Rusu MC, Pop F, Curcă GC, Podoleanu L, Voinea LM. The pterygopalatine ganglion in humans: a morphological study. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2009 Apr:191(2):196-202. doi: 10.1016/j.aanat.2008.09.008. Epub 2008 Nov 14
[PubMed PMID: 19124232]
[5]
Adameyko I, Fried K. The Nervous System Orchestrates and Integrates Craniofacial Development: A Review. Frontiers in physiology. 2016:7():49. doi: 10.3389/fphys.2016.00049. Epub 2016 Feb 19
[PubMed PMID: 26924989]
[6]
Ho KWD, Przkora R, Kumar S. Sphenopalatine ganglion: block, radiofrequency ablation and neurostimulation - a systematic review. The journal of headache and pain. 2017 Dec 28:18(1):118. doi: 10.1186/s10194-017-0826-y. Epub 2017 Dec 28
[PubMed PMID: 29285576]
Level 1 (high-level) evidence
[7]
Candido KD, Massey ST, Sauer R, Darabad RR, Knezevic NN. A novel revision to the classical transnasal topical sphenopalatine ganglion block for the treatment of headache and facial pain. Pain physician. 2013 Nov-Dec:16(6):E769-78
[PubMed PMID: 24284858]
[8]
Khonsary SA, Ma Q, Villablanca P, Emerson J, Malkasian D. Clinical functional anatomy of the pterygopalatine ganglion, cephalgia and related dysautonomias: A review. Surgical neurology international. 2013:4(Suppl 6):S422-8. doi: 10.4103/2152-7806.121628. Epub 2013 Nov 20
[PubMed PMID: 24349865]
[9]
Jürgens TP, May A. Role of sphenopalatine ganglion stimulation in cluster headache. Current pain and headache reports. 2014 Jul:18(7):433. doi: 10.1007/s11916-014-0433-4. Epub
[PubMed PMID: 24880803]
[10]
Elsås T, Edvinsson L, Sundler F, Uddman R. Neuronal pathways to the rat conjunctiva revealed by retrograde tracing and immunocytochemistry. Experimental eye research. 1994 Jan:58(1):117-26
[PubMed PMID: 8157097]
[11]
Tepper SJ, Caparso A. Sphenopalatine Ganglion (SPG): Stimulation Mechanism, Safety, and Efficacy. Headache. 2017 Apr:57 Suppl 1():14-28. doi: 10.1111/head.13035. Epub
[PubMed PMID: 28387016]
[12]
Rusu MC, Pop F. The anatomy of the sympathetic pathway through the pterygopalatine fossa in humans. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2010 Feb 20:192(1):17-22. doi: 10.1016/j.aanat.2009.10.003. Epub 2009 Nov 5
[PubMed PMID: 19939656]
[13]
Lovasova K, Sulla IJ, Bolekova A, Sulla I, Kluchova D. Anatomical study of the roots of cranial parasympathetic ganglia: a contribution to medical education. Annals of anatomy = Anatomischer Anzeiger : official organ of the Anatomische Gesellschaft. 2013 May:195(3):205-11. doi: 10.1016/j.aanat.2013.01.011. Epub 2013 Feb 4
[PubMed PMID: 23433588]
[14]
Espinosa-Medina I, Outin E, Picard CA, Chettouh Z, Dymecki S, Consalez GG, Coppola E, Brunet JF. Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science (New York, N.Y.). 2014 Jul 4:345(6192):87-90. doi: 10.1126/science.1253286. Epub 2014 Jun 12
[PubMed PMID: 24925912]
[15]
Dyachuk V, Furlan A, Shahidi MK, Giovenco M, Kaukua N, Konstantinidou C, Pachnis V, Memic F, Marklund U, Müller T, Birchmeier C, Fried K, Ernfors P, Adameyko I. Neurodevelopment. Parasympathetic neurons originate from nerve-associated peripheral glial progenitors. Science (New York, N.Y.). 2014 Jul 4:345(6192):82-7. doi: 10.1126/science.1253281. Epub 2014 Jun 12
[PubMed PMID: 24925909]
[16]
Hara H, Jansen I, Ekman R, Hamel E, MacKenzie ET, Uddman R, Edvinsson L. Acetylcholine and vasoactive intestinal peptide in cerebral blood vessels: effect of extirpation of the sphenopalatine ganglion. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1989 Apr:9(2):204-11
[PubMed PMID: 2921295]
[17]
Talman WT, Nitschke Dragon D. Neuronal nitric oxide mediates cerebral vasodilatation during acute hypertension. Brain research. 2007 Mar 30:1139():126-32
[PubMed PMID: 17291465]
[18]
Toda N, Ayajiki K, Yoshida K, Kimura H, Okamura T. Impairment by damage of the pterygopalatine ganglion of nitroxidergic vasodilator nerve function in canine cerebral and retinal arteries. Circulation research. 1993 Jan:72(1):206-13
[PubMed PMID: 8417843]
[19]
Roloff EV, Tomiak-Baquero AM, Kasparov S, Paton JF. Parasympathetic innervation of vertebrobasilar arteries: is this a potential clinical target? The Journal of physiology. 2016 Nov 15:594(22):6463-6485. doi: 10.1113/JP272450. Epub 2016 Oct 5
[PubMed PMID: 27357059]
[20]
Mojica J, Mo B, Ng A. Sphenopalatine Ganglion Block in the Management of Chronic Headaches. Current pain and headache reports. 2017 Jun:21(6):27. doi: 10.1007/s11916-017-0626-8. Epub
[PubMed PMID: 28432602]
[21]
May A, Goadsby PJ. The trigeminovascular system in humans: pathophysiologic implications for primary headache syndromes of the neural influences on the cerebral circulation. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism. 1999 Feb:19(2):115-27
[PubMed PMID: 10027765]
[22]
Goadsby PJ, Edvinsson L. Human in vivo evidence for trigeminovascular activation in cluster headache. Neuropeptide changes and effects of acute attacks therapies. Brain : a journal of neurology. 1994 Jun:117 ( Pt 3)():427-34
[PubMed PMID: 7518321]
[23]
Alfieri A, Jho HD, Schettino R, Tschabitscher M. Endoscopic endonasal approach to the pterygopalatine fossa: anatomic study. Neurosurgery. 2003 Feb:52(2):374-78; discussion 378-80
[PubMed PMID: 12535367]
[24]
Siéssere S, Vitti M, Sousa LG, Semprini M, Iyomasa MM, Regalo SC. Anatomic variation of cranial parasympathetic ganglia. Brazilian oral research. 2008 Apr-Jun:22(2):101-5
[PubMed PMID: 18622477]
[25]
Iwanaga J, Wilson C, Simonds E, Vetter M, Schmidt C, Yilmaz E, Choi PJ, Oskouian RJ, Tubbs RS. Clinical Anatomy of Blockade of the Pterygopalatine Ganglion: Literature Review and Pictorial Tour Using Cadaveric Images. The Kurume medical journal. 2018 Dec 21:65(1):1-5. doi: 10.2739/kurumemedj.MS651001. Epub 2018 Aug 30
[PubMed PMID: 30158355]
[26]
Goadsby PJ, Lipton RB. A review of paroxysmal hemicranias, SUNCT syndrome and other short-lasting headaches with autonomic feature, including new cases. Brain : a journal of neurology. 1997 Jan:120 ( Pt 1)():193-209
[PubMed PMID: 9055807]
Level 3 (low-level) evidence
[27]
Goadsby PJ. Pathophysiology of cluster headache: a trigeminal autonomic cephalgia. The Lancet. Neurology. 2002 Aug:1(4):251-7
[PubMed PMID: 12849458]
[28]
. Headache Classification Committee of the International Headache Society (IHS) The International Classification of Headache Disorders, 3rd edition. Cephalalgia : an international journal of headache. 2018 Jan:38(1):1-211. doi: 10.1177/0333102417738202. Epub
[PubMed PMID: 29368949]