Introduction
The nuclear renal scan, also known as renal scintigraphy, is an imaging method that uses radiopharmaceuticals/radiotracers to evaluate renal anatomy, physiology, and pathology. The various injected radiotracers are extracted from the blood by the kidneys and emit radiation, particularly gamma radiation, that is detected by gamma cameras. These studies are easy to perform and typically do not require sedation.
The use of nuclear scintigraphy has evolved over the last decades. Use has been affected by
- Improvement in ultrasound, computed tomography (CT), and magnetic resonance (MR) imaging
- Development and availability of various radionuclides
- Current standard and preferred methods for the workup of various conditions
Anatomy and Physiology
The kidneys are located bilaterally at the upper lumbar vertebra with the left kidney typically slightly superior in location than the right kidney.
Each kidney can be divided into renal parenchyma and renal sinus.
Renal parenchyma consists of the peripheral renal cortex and the more central renal medulla. The outer cortex contains the glomeruli, peritubular capillaries, renal tubules, and cortical portions of the collecting ducts that form urine. The renal medulla contains the medullary portion of the collecting ducts which completes urine concentration begun at the glomeruli. Urine drains into the renal calyces.
The renal sinus consists of calyces, the renal pelvis, and the renal arteries and veins. The renal pelvis is a funnel-shaped structure, which connects the renal calyces to the ureter(s) which descend to the bladder.
The kidney's blood supply is derived from the renal arteries, originating laterally off the abdominal aorta. The renal arteries divide into smaller and smaller branches, progressing into segmental, interlobar, arcuate, and interlobular arteries. The interlobular arteries eventually become the even smaller afferent arterioles and then the glomerular capillaries, that supply the glomeruli. The glomerular capillaries act as semi-permeable membranes that help produce 20% of the urine via passive filtration of the plasma. The renal tubules produce the remaining 80% of the urine via active tubular secretion. The glomerular capillaries beyond the glomeruli become efferent arterioles leading to peritubular capillaries that surround renal tubules and help reabsorb and concentrate urine while retaining various electrolytes.
Nuclear scintigraphy and its various agents take advantage of the aforementioned renal anatomy and physiology. Injected radiopharmaceuticals enter the renal artery and eventually enter renal glomeruli. Radiotracers primarily filtered by glomeruli are useful to evaluate glomerular filtration rates, which is a way to evaluate renal function. Radiotracer secreted by the tubules is used to assess the estimated renal plasma flow. Renal cortical agents predominantly bind to renal tubules within the kidney cortex and allow renal anatomic evaluation (see discussion below).
Indications
Renal scintigraphy usage and indications have changed with the improvements in other modalities particularly regarding each modality's ability to resolve kidney anatomy and evaluate the kidneys for the disease. Despite improvements in anatomic resolution by various other modalities lessening the role of scintigraphy for anatomic evaluation, scintigraphy maintains a key role in evaluating renal function. It has a role in evaluating renal, pylocalyceal, ureteral, and bladder anatomy.
Areas of clinical evaluation with nuclear medicine agents include:
- Reno-vascular hypertension
- Obstructive uropathy
- Renal anomalies
- Renal transplant
- Renal parenchymal infections particularly pyelonephritis and postinfectious scarring
- Renal masses
- Renal trauma
- Ureteric trauma
Contraindications
Pregnancy is an absolute contraindication for the renal scan so as to prevent radiation exposure to the fetus.
Tc-99m labeled radiopharmaceuticals are used for renal scanning which can be excreted in breast milk. According to the Academy of Breastfeeding Medicine (ABM), nuclear scintigraphy agents do not require breastfeeding interruption since their effective dose to the infant falls below 1 millisievert (mSvt).[1][2] However, a 4 hours cessation of breastfeeding for 99mTc-MAG3 and a 12- hour interruption for 99mTc-DTPA is an accepted safety methodology to reassure mothers.[3]
Equipment
Gamma scintillation camera systems are used for renal scanning. These systems convert radiotracer emitted photons from the radiopharmaceuticals into display images.
The gamma camera system consists of a collimator, scintillation crystal, photomultiplier tubes (PMTs), and a processing computer.
A collimator is placed between the patient and the gamma camera. It localizes the radiated area to only the required field of view for the scan absorbing undesired radiation and that outside the field of view from reaching the gamma cameras. The scintillation crystals convert emitted gamma rays from the patient into a florescent light pulse. Photomultiplier tubes convert the light pulse into a signal of various voltages. The gamma camera system's computer converts the output signal into an image based on how the radiopharmaceutical has been distributed. The gamma camera system's computer is used to further refine and format the images for interpretation. Quantitative information can be determined. That information and the resultant images are then placed into a local or hospital-wide PACS (picture archiving and communications) system.
Single-photon emission computed tomography (SPECT) systems fuse information obtained from gamma cameras with anatomy determined by CT.
Personnel
Nuclear medicine technologists are certified to perform renal scintigraphy under the guidance of a supervising radiologist or nuclear medicine specialist.
Preparation
The patients undergoing renal scanning should be well hydrated so as to minimize the radiation dose to the bladder wall which would be increased with a collapsed bladder. Dehydration can delay renal excretion and interfere with the study results.
Most renal scintigraphy requires no premedication or dietary restriction.
Some specialized renal scans however have preparation.
In diuretic renography, which is performed for evaluation of obstructive uropathy prostaglandins act as vasodilators of the afferent arterioles of glomeruli and help to maintain glomerular filtration rates by regulating renal blood flow. Non-steroidal anti-inflammatory drug (NSAID) such as diclofenac decrease prostaglandin synthesis and result in decreased renal perfusion as well as glomerular filtration which thereby affects glomerular filtration rate. A study on experimental animals proved that intravenous administration of diclofenac delays renal excretion, especially in renography using radiotracers primarily filtering through glomeruli. NSAID also affects ureteric contraction and delays excretion.[4] Diclofenac and other NSAIDs are therefore avoided on the day of study.
In angiotensin-converting enzyme inhibitor (ACEI) renography which is used to evaluate renal hypertension, captopril is given orally before the study. The presence of food in the gastro-intestinal tract can delay the absorption of the drug. Fasting at least 4 hours before this study is recommended. If the patient is already taking ACEI, it should be stopped before the study. Captopril should be stopped at least 48 hours before and lisinopril or enalapril stopped at least 1 week before ACEI renography examination. Long term usage of ACEI reduces the sensitivity of the test.[5]
Technique or Treatment
Patients are instructed to void just before their renal scan since a full bladder delays upper urinary tract emptying and interferes with the study results reliability especially when working up a case of obstructive uropathy. Occasionally foley catheterization is used to keep the bladder empty. Patients are pre-hydrated before tracer injection and post hydrated after injection.
The choice of radiopharmaceutical used is related to the type of renal scan being performed.
The various radiotracers agents used for renal scintigraphy include:
1- Technetium-99m diethylene triamine penta-acetic acid (Tc-99m DTPA): Primarily cleared by glomerular filtration and therefore useful to measure GFR and to evaluate flow through the pyelocalyceal system through the ureter and into the bladder. Dose: Up to 15 Millicurie (mCi) for adults and 0.1-0.2 mCi/kg for children with a maximum pediatric dosage of 5 mCi.
- Technetium-99m dimercaptosuccinic acid (Tc-99m DMSA): This agent predominantly binds to renal tubular cells in the renal cortex allowing cortical imaging. Because it is bound to the cortex for a long time (40% of dose concentrated in kidneys at 6 hours post-injection), it is primarily used for cortical anatomy and assessment of pathologies such as renal ectopia or renal scarring. However, because of its effective long half-life of 6 hours in the kidneys, exposing the kidneys to greater radiation exposure than other agents, many people use technetium-99m mercaptoacetyl triglycine (Tc-99m MAG3) for cortical imaging. Of note, DMSA also is disadvantaged by a short shelf life after preparation. Dose: 5 mCi in adults and 0.05 mCi/kg for children with a maximum pediatric dosage of 2.7 mCi.
- Technetium-99m mercaptoacetyl triglycine (Tc-99m MAG3): This agent is secreted predominantly by proximal renal tubules with a small component (approximately 5%) of it filtered by the glomeruli. Dose: Up to 10 mCi for adults and 0.15 mCi/kg for children with a maximum pediatric dosage of up to 4 mCi. Tc-99m MAG3 is extracted from plasma with greater efficiency resulting in retained parenchymal activity and allowing renal parenchyma evaluation.[6] MAG3 studies also allow for the assessment of urine flow through the pyelocalyceal system and bladder. It has 40% more plasma clearance than that of Tc-99m DTPA improving imaging.[7] Although it has more limited cortical anatomy evaluation than Tc-99m DMSA it allows additional information regarding function (a less practical possibility with Tc-99m DMSA its longer half-life).
Radiotracers are given by bolus injection and patients are then placed under the gamma camera. For native kidney function evaluation, images are acquired of the patient, posteriorly. Anterior image acquisition is usually performed for renal transplant evaluation, as renal grafts are usually placed in the anterior iliac fossa. The scintillation gamma cameras placed below the patient convert emitted radiation into a displayed image (see equipment section).
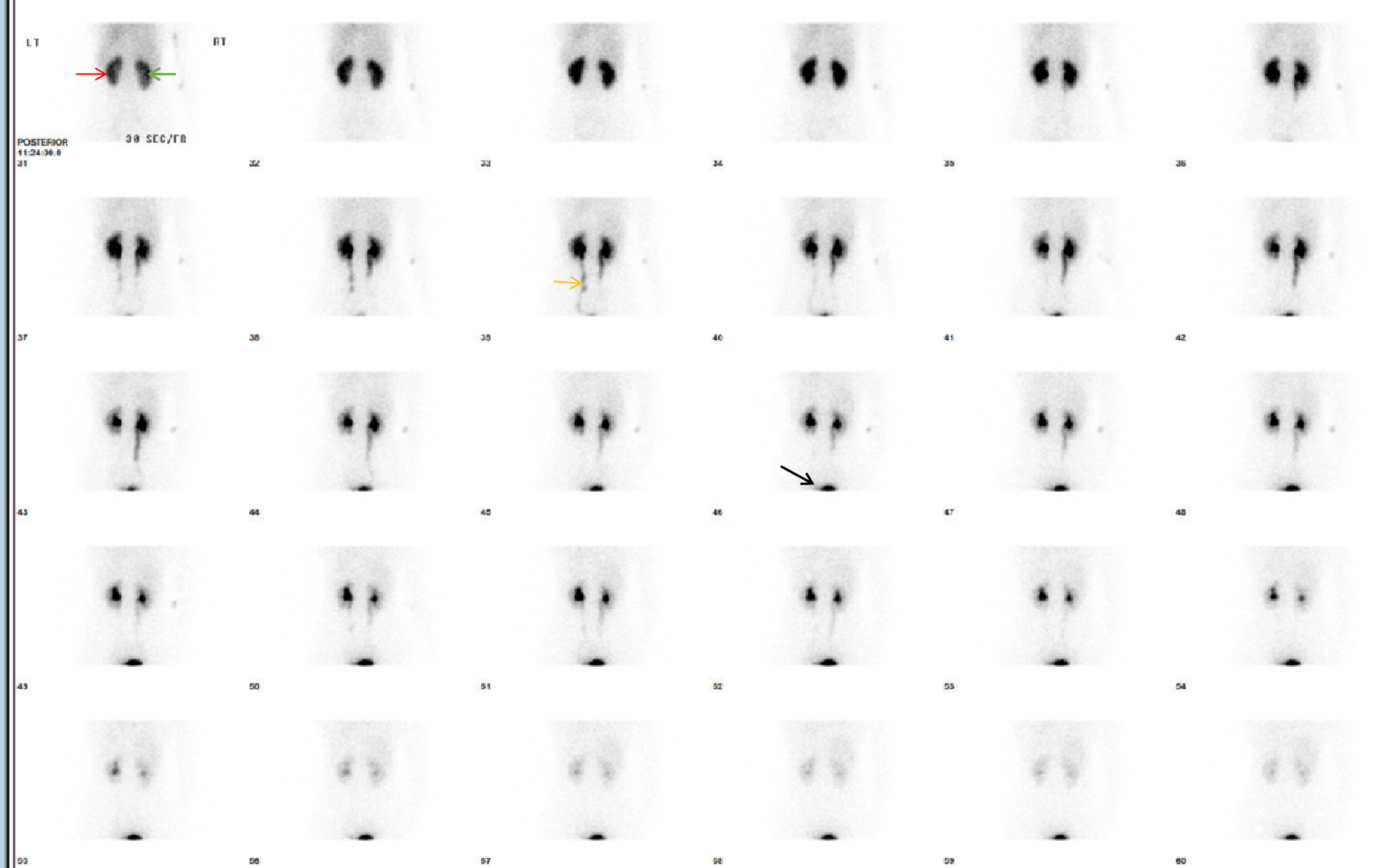
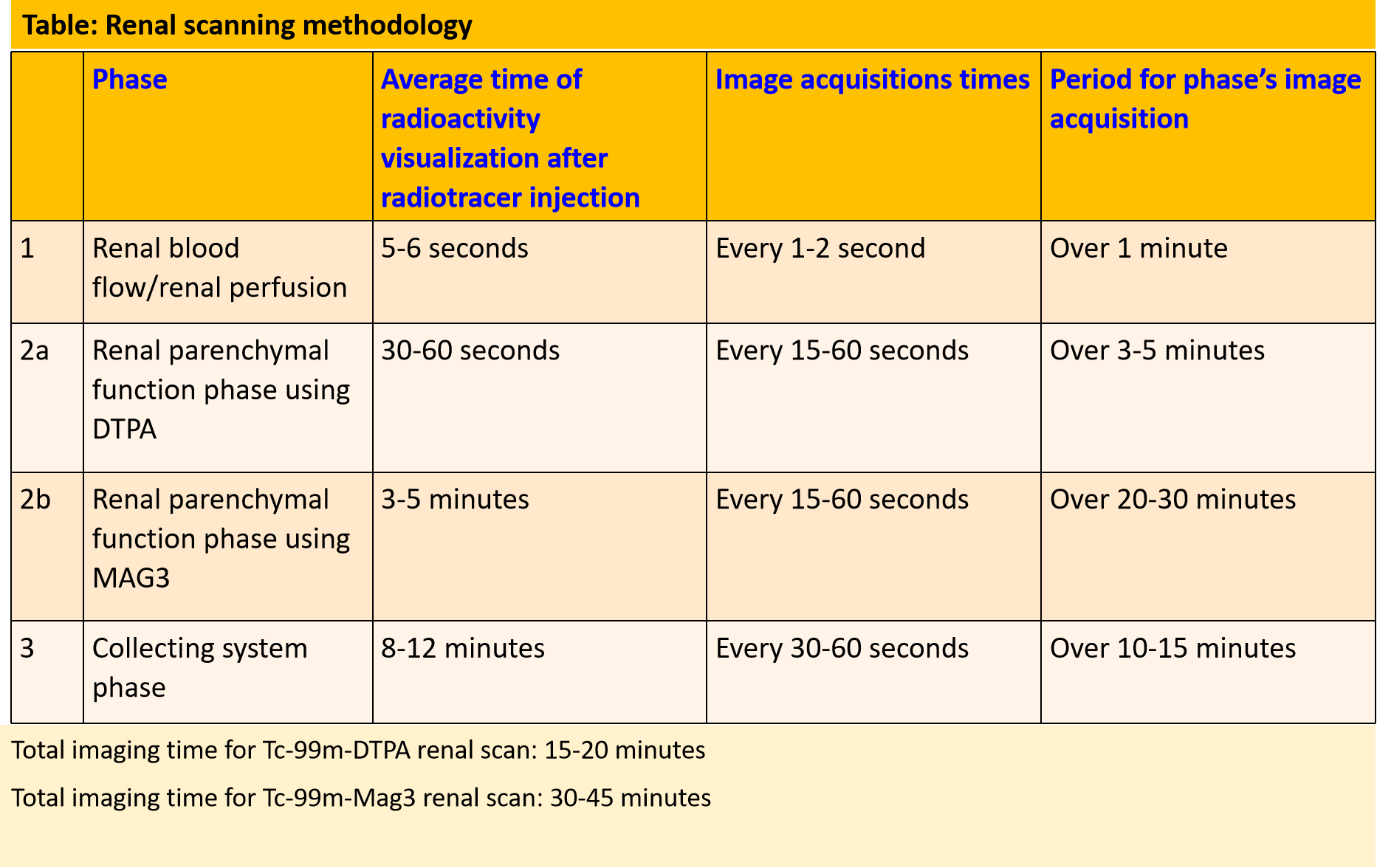
Serial static or dynamic image acquisitions are set up at different times during the study with the exact technique depending on the study being performed. A bolus of radionuclide placed in an antecubital vein typically reaches the renal arteries from the aorta within 1 second. For renal blood flow evaluation, serial dynamic images are acquired every 1 to 2 seconds for up to a minute. The kidneys are usually seen by 5 to 6 seconds from injection and show maximal activity in normal patients by 30 to 60 seconds. To evaluate renal parenchymal function, a series of images are obtained after 1 minute of the renal perfusion phase. Images are acquired at 15-60 seconds intervals for a period of 3-5 minutes when using 99mTc-DTPA and for 20-30 minutes when using 99mTc-MAG3. This phase allows for the best imaging of renal anatomy via parenchymal uptake. Radiotracer activity usually starts appearing in the collecting system or urinary bladder by 4-8 minutes. Collecting system evaluation is accomplished after ambulating to promote pyelocalyceal system drainage or after a diuretic (Lasix dosage is dependent on age and renal function) is given. An indwelling open bladder catheter is required for patients with urinary bladder non-compliance or difficulty in urination, as a full bladder limits/delays upper urinary tract emptying.
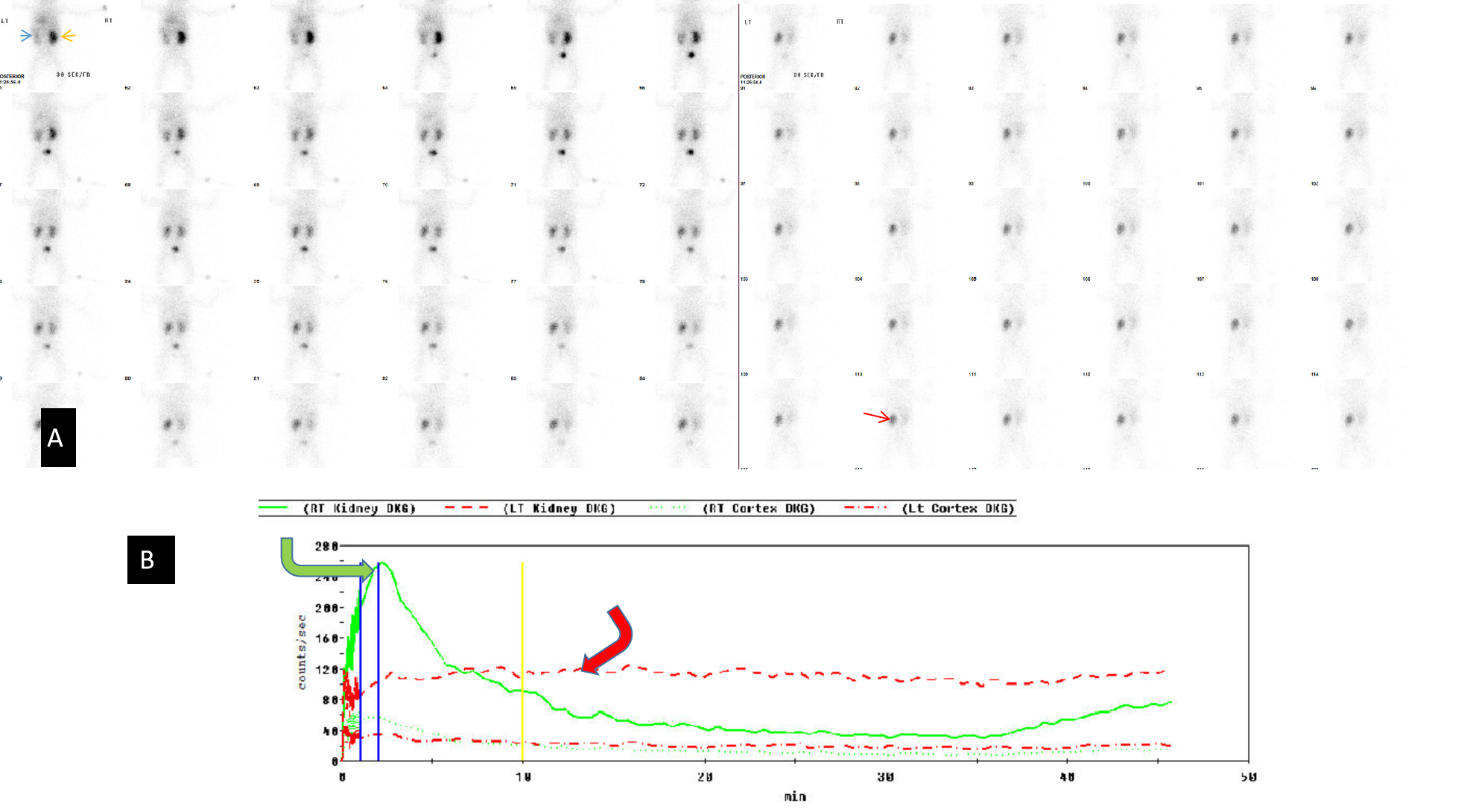
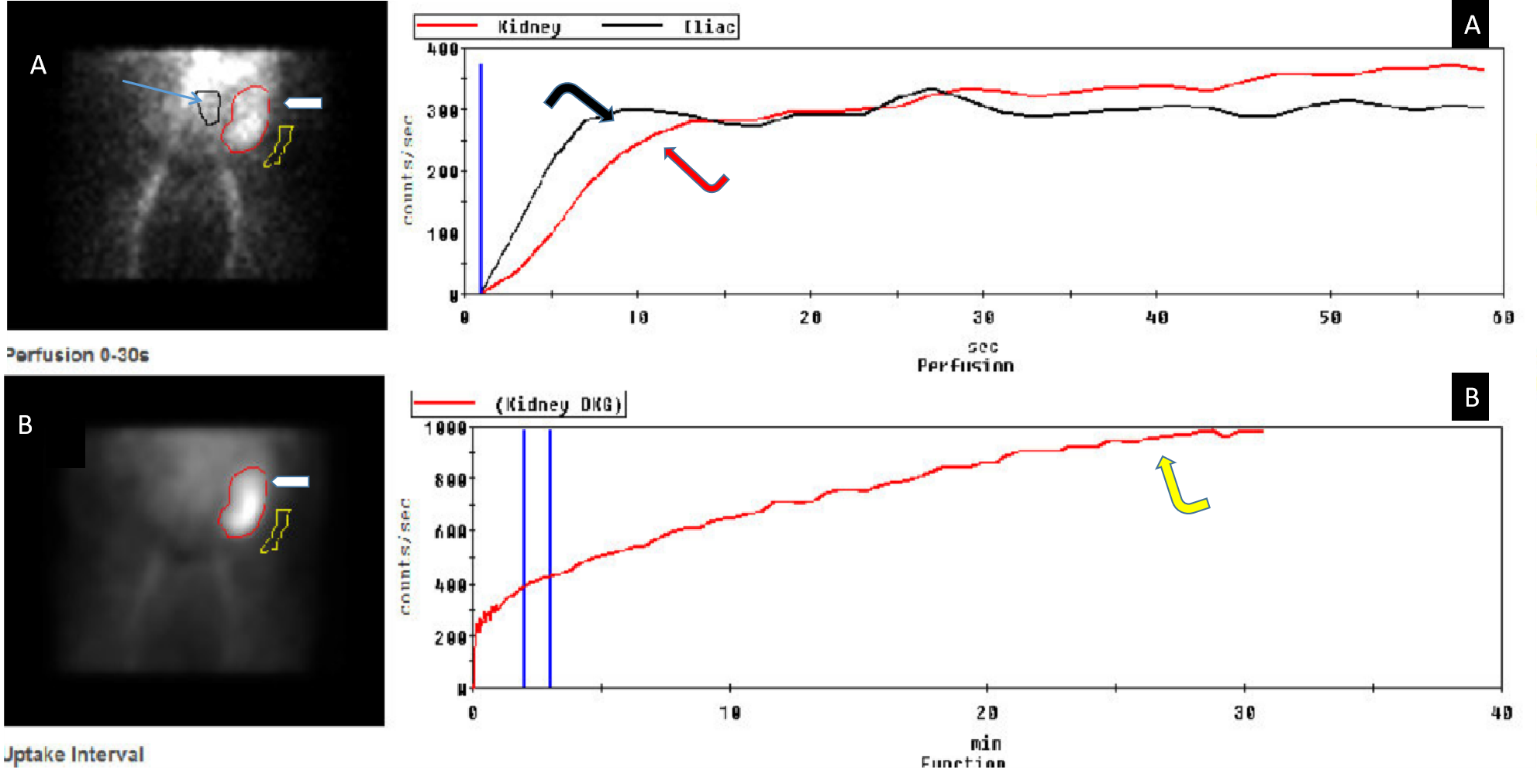
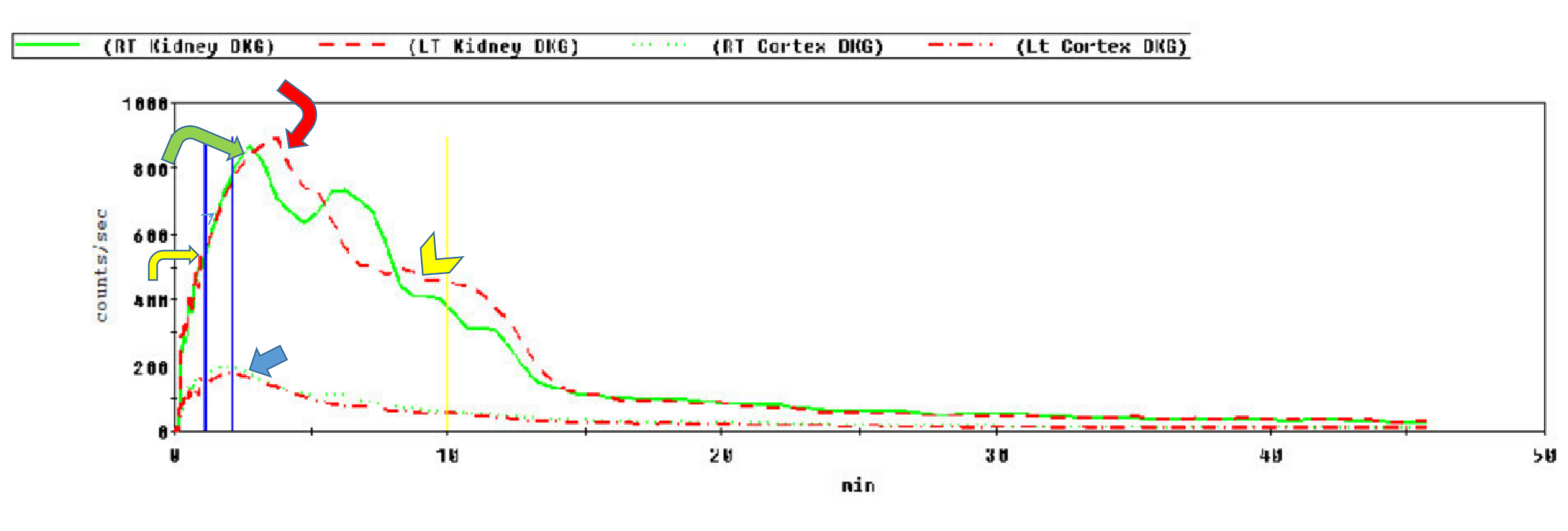
Renal anatomy and function are assessed visually from the acquired images. In addition, a time-activity curve is generated based on the series of images, and renal function is quantified from the time-activity curve in e.g. Tc-99m MAG3 renal scanning. Time activity curves are generated by the system's computer based on the assessment of radionuclide activity over a region of interest (ROI). For renograms, the ROI is placed over each individual kidney. Radiotracer activity beyond the kidneys is subtracted out of the image. Information is graphically displayed to about 20 to 30 minutes after radiotracer injection. A typical renogram has 3 phases. The first phase is known as the vascular transit phase which represents radiotracer entering the kidneys. It usually lasts about 30 to 60 seconds. The second phase is known as the tubular concentration phase or parenchymal transit phase normally lasts 1 to 5 minutes when radiotracer appears in the tubules. It is represented by a peak in the renogram. The third phase is noted by a downslope in the renogram indicating excretion of the radiotracer from the kidneys and clearance from the collecting system. It usually starts at 4-8 minutes after radiotracer injection (See image- 3).
For accurate renal perfusion assessment, a different time activity curve can be created. The ROI is placed on each kidney but is compared with a time-activity curve obtained from the aorta. Entry of the radiopharmaceutical into the aorta is shown as an upstroke in the aortic flow curve. It is usually seen at 30 to 60 seconds after injection. Flow into each kidney is assessed in relationship to flow in the aorta. A flat curve would suggest abnormal flow from the aorta to the kidney.
As a note, when evaluating renal cortical function, the ROI should be restricted to the renal cortex and excluding renal pelvic activity, as the retained radiotracers in the renal pelvis confuse the renal cortical assessment.[8]
Various semiquantitative data such as time to peak activity (Tmax), relative uptake ratios, half time excretion (T1/2), and 20-minute time to peak count ratios (20 min/maximum count ratio) can be obtained from the renogram.
Diuretic renography: Diuretic renography is used for the assessment of urinary tract obstruction. It can distinguish between genuine obstruction and non-obstructive atonicity of the ureter that occurs with vesico-ureteric reflux, congenital malformations, or a non-compliant urinary bladder.[9] O’Reilly noted that diuretics should be used only if the baseline renogram shows a delay in excretion.[10] The diuretic such as furosemide is administered 15 to 20 minutes after radiotracer injection to facilitate radiotracer excretion from the renal pelvis and ureter. Additional imaging beyond the aforementioned anatomy images (see table) are acquired every 15 to 60 seconds for another 20 to 30 minutes.
When radiotracer excretion increases after the diuretic, the exam is interpreted as non-obstructive dilation. If after given a diuretic excretion is unaffected or shows little change, the diagnosis is obstructive uropathy. In obstructive uropathy, the renogram demonstrates continuous rising and little or no downsloping during the excretion phase (see image-4). A renogram may be flattened at the perfusion phase in cases of high grade-long standing obstruction which often leads to a decrease in renal perfusion. A T1/2 calculation obtained from the renogram represents radiotracer activity falling to 50% of its maximum value. It is calculated from the time of furosemide injection.[11] A study by Rossleigh et al showed normal furosemide half clearance using Tc-99m MAG3 should be less than 9.8 minutes.[12] A clearance half time of more than 20 minutes usually indicates obstruction.[13] However prolonged T1/2 should not be the sole criteria for the diagnosis of obstruction. Rather the diagnosis should be made from a combination of the scintigraphic images, the renogram as well as information obtained from other diagnostic modalities.[14]
Gravity assisted diuresis (GAD) (keeping the patient, often a child, in an upright position for 5 minutes after the renal scan to obtain further tracer drainage) often helps to confirm a diagnosis of true ureteropelvic junction (UPJ) junction obstruction. when T1/2 is in the grey zone of 10 to 20 minutes. If more than 50% of the quantifiable residual radio-tracer activity is present after GAD, true obstruction is confirmed.[13]. GAD also helps in assessing post pyeloplasty renal pelvis dilation for true obstruction. Post-surgical hypotonia of the renal pelvis which may result in pyelectasis and can have T1/2 greater than 20 minutes will be seen as having a lesser T1/2 and therefore suggesting normalcy/no obstruction after GAD. [13]
Angiotensin-converting inhibitor (ACEI) renal scintigraphy: ACEI renal scintigraphy is used for renovascular hypertension evaluation. ACEI induced renography has 90% sensitivity and specificity for the diagnosis of renovascular hypertension in patients with normal renal function including GFR.[14] Anatomic stenosis of the renal artery results in renal hypoperfusion and it is compensated for by angiotensin-converting enzyme (ACE) activation. This compensation leads to normal GFR and normal renal scan. When Angiotensin-converting inhibitor (ACEI) is administered, this compensation is eliminated and results in a diminution of glomerular filtration. On scintigraphy, this is noted by decreased renal uptake and excretion. Renography findings are most distinguishable when there is at least 60 to 90 % renal artery stenosis. Criteria for a unilateral stenosis diagnosis include less than 40% total uptake by a single kidney at 2-3 minutes and more than 2 minutes delay in time to peak (Tmax) when compared to the normal side.
Over the last decades, CT and MR angiography have proven to be more useful modalities for renal arterial flow analysis as they provide renal artery anatomic information as well. ACEI renography is useful for the assessment of renovascular hypertension in patients with renovascular hypertension that has ACE compensation. It cannot detect anatomic renal artery stenosis if such compensation has not occurred. Its importance is questionable in cases of bilateral renal artery stenosis.[5][14][15].
DMSA renography: Tc-99m DMSA demonstrates homogeneous cortical uptake, remaining bound to the cortical tubules for at-least 6 hours. The exam is simply a parenchymal imaging scan. High-resolution pinhole collimators are used for better delineation of the cortex.
DMSA renography is highly sensitive and has proven more helpful than ultrasonography for the early diagnosis of pyelonephritis in children over 5 years of age. Early detection and timely treatment of pyelonephritis are important in preventing complications such as cortical scarring and its associated loss of renal function.[16][5] The methodology is chosen in children to decrease radiation exposure compared to a CT scan. Acute pyelonephritis is not usually worked up in adults. CT is used for complicated cases.
Imaging findings of acute pyelonephritis include defects in cortical uptake of 99mTc-DMSA which can be focal or multifocal. Sometimes acute pyelonephritis presents as diffusely decreased activity due to renal edema. A bulging cortex may be noted in diffuse acute pyelonephritis.[5]
Renal cortical scarring is a common complication occurring after pyelonephritis and vesicoureteric reflux (VUR). DMSA scintigraphy shows defects in cortical uptake at the region of the scars with associated parenchymal volume loss. It is highly sensitive in detecting post VUR scarring in children less than 2 years of age who presents with febrile urinary tract infection. Some work with ultrasound contrast agents has shown another method, without radiation, to evaluate renal scarring.[17] Dilating VUR, defined as grade III-IV reflux, is considered a significant risk for renal scarring/persistent renal damage. DMSA helps to identify renal damage. A normal DMSA scan in such cases excludes the possibility of dilating (grade III-IV) VUR and some suggest it should be considered before ordering a voiding cystourethrogram (VCUG) study.[18][19][20]
DMSA scintigraphy is sometimes used to evaluate functioning parenchyma related to a renal mass. Nonfunctioning parenchymal masses e.g. renal neoplasm, hematoma, abscess, cyst, or infarcts appear as photopenic areas in DMSA scintigraphy. CT sonography and MRI have become more useful for renal mass evaluation.
Renal transplant evaluation: Renal scanning with 99mTc-MAG3 is used to evaluate function, complications, and treatment monitoring of renal transplant.[21] Scintigraphy is performed shortly after surgery for baseline function analysis with followup renal scintigraphy imaging if there is any concern regarding the transplant e.g. deterioration of function or postsurgical complication.
Early postoperative evaluation of time to peak in 99mTc-MAG3 clearance is used to predict renal cadaveric graft survival.[22] Normal perfusion demonstrates radiotracer activity in the graft. It is assessed in relation to the iliac vessels which feed the typical transplant.
Acute tubular necrosis (ATN) is one of the complications, which usually occurs during the first 3 to 4 days after transplantation. ATN usually develops because of ischemia of the transplant after harvesting and before transplantation. This is most frequently seen in cadaveric transplants. Scintigraphy shows the diminished renal function and cortical retention on 99mTc- MAG3 scintigraphy.
Renal transplant toxicity from the antirejection drug cyclosporin demonstrates similar findings of normal uptake and increased cortical retention with little or no excretion due to poor tubular function.
Post-surgical complications include leakage of urine and urinoma development. Urinomas are seen as accumulations of radiotracer adjacent to the point of leakage and beyond the expected location of the renal graft, ureter, and bladder. In hyperacute transplant rejection, renal scanning demonstrates absent renal perfusion and function within 0-24 hours of surgery. It occurs due to venous thrombosis secondary to the recipient's pre-formed antibodies to a foreign body, in this case, the transplant. Acute rejection usually occurs in 2 to 3 months due to cell-mediated lymphocytic infiltration. Nuclear scanning shows decreased renal perfusion as well as radionuclide uptake and excretion. Chronic rejection is an antibody-mediated process occurring beyond 3 months to years after surgery. Nuclear scanning, in such cases, shows gradual deterioration of transplant perfusion and function.[5][21]
Complications
Complications for nuclear renal scans are minimal. They include the potential complication of radiation exposure to patients, but exposures are very low compared to for example a CT scan of the abdomen. Radiation exposure from a 99m-Tc labeled renal scan is approximately 1 msvt as compared to 10 msvt for a CT scan of the abdomen. According to a 2006 article, the radiation dose of single technetium 99m nuclear renal scan was approximately equivalent to the exposure from 50 chest radiographs while that of an abdominal or pelvic CT was reported as equivalent to 500 chest films.[23] Certainly, radiation dosages for CT have improved over the last decade with such techniques as iterative reconstruction being introduced.
Pain or inflammation can develop at the injection site as with any injection.
Adverse reactions from radionuclides include vasovagal reaction, fever, and allergies, but most of them are mild and do not require hospitalization.[24]
Clinical Significance
Renal scintigraphy is used for evaluation of renal perfusion, and function as well as renal anatomy. As CT and MRI imaging has advanced the role of renal scintigraphy has changed. Currently, it is usually limited to renal perfusion and function as anatomy evaluation is predominantly done by ultrasound, CT scan, and MRI. Regarding anatomy, renal scintigraphy is currently used when there is an allergy to CT or MRI contrast material. The use of IV contrast in CT, as well as MR, is avoided in cases of abnormal renal function and altered GFR. Nuclear scanning agents can be used in such cases. Renal scintigraphy is also preferred in patients with claustrophobia who cannot undergo CT or MRI exams.
Renal scintigraphy has a role in the diagnosis of obstructive uropathy. It can be used to differentiate true obstruction from nonobstructive simulators causing urinary tract dilation.
A captopril study can diagnose compensated unilateral renal hypertension. DMSA cortical scintigraphy is useful in detecting renal scar or acute pyelonephritis specifically in children where there is higher sensitivity than routine ultrasound and lower radiation than CT scan. However, contrast-enhanced ultrasound is an emerging modality that has been used to identify post vesicoureteral reflux renal scarring.
Nuclear renal scanning is an excellent modality for the qualitative as well as quantitative assessment of renal transplant function. It is useful in post-transplant complication detection. The early detection of function in a renal transplant allows early management decisions including, when irreversible, removal of the transplant. Timely removal of a damaged transplanted kidney can save a patient from unnecessary immunosuppression administration.[25]
Because radiation exposure from renal scintigraphy is very low as compared to a CT scan, it maintains a role in the evaluation of pediatric renal anatomy whether normal, anomalous, or pathologic.
Enhancing Healthcare Team Outcomes
Renal scintigraphy requires coordination and interprofessional communication between radiologists, nuclear medicine physicians, nuclear medicine technologists, and the treating physicians and/or surgeons so as to provide well done and appropriate studies.
A review of the patient's history and physical examination and prior diagnostic studies are necessary to help plan the type of nuclear scanning that may be needed to be performed. The clinical note requesting such an exam should indicate the clinical question that needs to be answered. The interpreting radiologist/nuclear medicine physicians should review all available clinical, radiological, and laboratory data as well as current medications before performing the study. Special attention must be made for any patient with a history of fluid restriction, reduced urine volume, or current use of diuretics since these situations can affect the nuclear scan and its interpretation.
Renal scintigraphy (like all nuclear medicine exams) requires cooperation between the pharmacist, nuclear medicine technologist, nurses, registered radiology assistants as well as a radiologist or nuclear medicine specialist for proper radiotracer administration and image timing.
The above statements regarding renal scintigraphy are part of standard radiographic practice. (see ACR–SPR practice parameter for the performance of renal scintigraphy).
Radiation safety is the responsibility of all involved staff. The radiation dose in diagnostic procedures should be maintained as per the “as low as reasonably achievable” (ALARA) principle. A practice guideline was developed in 2010, by an expert panel including pediatric radiologists, nuclear medicine physicists, as well as nuclear medicine specialists and nuclear medicine technologists to optimize radiation doses for children.[26] A study showed that the implementation of this guideline led to a positive effect on standardization and dose reduction in pediatric nuclear medicine. [27] (level 3).
Radiation safety is crucial in managing all patients. Though nuclear scanning procedures are not actually performed in pregnant patients, the agents are associated with minimal fetal radiation dosages (a few mSv).[28] Despite this, it is the responsibility of all involved personnel to know the pregnancy status of patients before doing a procedure involving radiation.
The results of renal scintigraphy should be properly communicated to the patient's physician (see ACR practice parameter for communication of diagnostic imaging findings).