Continuing Education Activity
Gastric Bypass is a common operation to assist patients with severe obesity with weight loss. This surgery was traditionally done with the open approach, however, this approach was associated with incidences as high as 20 percent of incisional hernias and wound complications as high as 8 percent. This led to the advent of the laparoscopic approach to improve postoperative outcomes in bariatric patients. The first case series of laparoscopic Roux-en-Y gastric bypass (RYGB) was presented in 1994 by Drs. Wittgrove and Clark. Much data has proven that the laparoscopic approach to RYGB results in decreased length of hospital stay, decreased intraoperative blood loss, less postoperative pain, fewer pulmonary complications, and fewer wound infections. This activity describes the indications, contraindications, and complications of laparoscopic gastric bypass surgery and highlights the role of the interprofessional team in the management of patients with obesity.
Objectives:
- Describe the indications for laparoscopic gastric bypass.
- Explain the technique involved in performing a laparoscopic gastric bypass.
- Describe the potential complications of a laparoscopic gastric bypass.
- Explain the importance of optimizing care coordination among interprofessional team members to improve outcomes for patients requiring bariatric surgery.
Introduction
Gastric Bypass is a common operation for weight loss in patients with severe obesity. The procedure was developed in the 1960s by Drs. Mason and Ito who observed significant weight loss in a patient undergoing partial gastrectomy for peptic ulcer disease. This surgery was traditionally done with the open approach, and as with any open surgery, there were incidences as high as 20% of an incisional hernia and wound complications as high as 8%. This led to the advent of the laparoscopic approach to improve postoperative outcomes in bariatric patients. The first case series of laparoscopic Roux-en-Y gastric bypass (RYGB) was presented in 1994 by Drs. Wittgrove and Clark, and the largest trial was reported by Nguyen and colleagues in 2001. Much data has proven that the laparoscopic approach to RYGB results in decreases in hospital stay, intraoperative blood loss, postoperative pain, pulmonary complications, and wound infections. Studies have shown a steep learning curve for the laparoscopic gastric bypass, and a possible increased rate of postoperative internal hernia (a surgical emergency.) Despite this, it is now considered safer and more cost-effective than traditional, open RYGB.
Today over 90% of gastric bypasses performed for weight loss are done laparoscopically. Despite being one of the most challenging, minimally invasive operations, it has become the most common foregut surgery performed in the United States. Of the many ways to perform this surgery, the fundamentals of each technique remain the same.
Anatomy and Physiology
To perform a laparoscopic gastric bypass, the clinician must have a thorough understanding of the entire intraabdominal cavity. There are many different organs involved, but this operation intimately involves the stomach, small intestine, liver, spleen, transverse colon and its mesentery, and diaphragm.
The stomach is perhaps the most important organ to understand anatomically. It is a muscular tube that generally makes a “reversed C” contour when viewed from anterior to posterior. It begins at the lower esophageal sphincter and ends as it continues as the first portion of the duodenum. It is divided into the cardia (just distal to GE junction), fundus (abutting the left diaphragm), body, antrum, and pylorus (most distal portion entering the duodenum). The lesser curvature lies beneath the medial segments of the liver and is attached to it via the gastrohepatic ligament (the lesser omentum). The spleen lies in the left upper quadrant and is attached to the greater curvature of the stomach by the gastrosplenic ligament (containing the short gastric vessels).
The duodenum is divided into four portions. The second portion of the duodenum contains the duodenal papilla which is the opening on the medial portion of the duodenum that allows the common bile duct and pancreatic duct to drain into the alimentary tract. The fourth portion of the duodenum emerges from the retroperitoneum and traverses the transverse mesocolon at the ligament of Treitz to become the jejunum. The jejunum lies in the intraperitoneal cavity and transitions into the ileum, which leads to the large intestine at the ileocecal valve. The average small bowel length is approximately 500 cm but can range from as little as 200 cm to about 800 cm.
Indications
There are few indications for laparoscopic gastric bypass in the modern age. In rare cases, a surgeon may consider this procedure for gastric outlet obstruction secondary to tumors or peptic ulcer disease. [1]
Main Indication for Bariatric Patients
For a patient to be a candidate for any weight loss surgery, they historically must meet the following criteria [2]:
- BMI greater than or equal to 40 or
- BMI greater than or equal to 35 with at least one obesity-related comorbid condition (HTN, DM, severely limiting MSK issues):
- Unsuccessful nonoperative weight loss attempts
- Mental health clearance
- No alcohol or substance abuse
- No medical contraindication to surgery
The most common bariatric surgery is the sleeve gastrectomy. Head-to-head comparisons in the recent literature between SG and RYGB and have shown comparable results in excess weight loss and resolution of comorbidities at 5 years postoperatively [3]. The major exception is gastroesophageal reflux disease, which has been shown to worsen in almost one-third of patients undergoing sleeve gastrectomy. A significant percentage of these patients may require conversion to RYGB due to unrelenting symptoms of GERD. This is a relative indication to choose laparoscopic gastric bypass over sleeve gastrectomy when considering weight loss surgery. [4]
Contraindications
The contraindications for this procedure are generally the same as with any laparoscopic procedure and include the inability to tolerate pneumoperitoneum, uncorrectable coagulopathy, and previous abdominal surgeries (relative c/i) [5]
Patients with conditions requiring long-term endoscopic surveillance, as well as Barrett esophagus with severe dysplasia, are both contraindications to laparoscopic gastric bypass.
Equipment
The basic laparoscopic equipment required for this operation includes insufflation with CO2, drapes, monitors, laparoscopic instruments, electrocautery, and trocars.
Contrary to conventional laparoscopic procedures, with bariatric patients, the procedure requires longer trocars as well as longer laparoscopic instruments to accommodate for the thicker abdominal wall.
There are many different techniques to perform a gastric bypass; however, many of the steps and equipment required are uniform:
- A total of 5 trocars are typical, ranging from 5 to 12 mm in size
- A liver retractor
- 10 mm, 30-degree angled laparoscope
- Endoscopic linear stapler; some methods require circular stapler
- Endoscope
- Ultrasonic energy device
Personnel
Patients are required to be seen by a an interprofessional team before being a bariatric surgery candidate. This includes a nutritionist, a psychiatric specialist, surgical team, and the primary care physician.
For the operative portion, the following personnel is required:
- Anesthesiologist
- Primary surgeon
- Scrub technician
- First assistant
- Circulating nurse
Preparation
Preoperatively, all patients should receive upper endoscopy with Helicobacter pylori testing, abdominal ultrasonography, pulmonary function tests, and basic laboratory evaluation.
The patient is given preoperative antibiotics 30 minutes before incision as well as venous thromboembolism prophylaxis. Abdominal hair is removed with clippers in the preoperative area. After induction of anesthesia, a Foley catheter is inserted, and an orogastric tube is positioned within the stomach.
Technique or Treatment
The following are common methods and variations that surgeons may perform [6]:
- Setup and entrance into the abdomen
- Creation of the Roux-limb
- Jejunojejunal anastomosis
- Creation of the gastric pouch
- Gastrojejunal anastomosis
- Endoscopy
- Closure
The patient is positioned supine on the operating table with the arms extended and placed on arm boards. The patient is secured to the table with multiple means (tape, velcro, spindle sheet on the patient's pelvis).
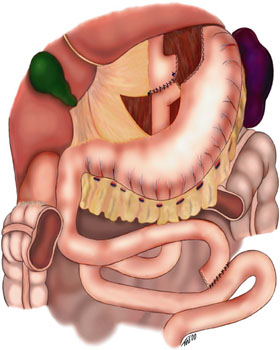
- Setup and entrance into the peritoneal cavity: The abdomen is prepped and draped in standard sterile fashion. The operating surgeon will stand on the patient's right side along with the scrub technician. The assistant will stand on the patients left side. Entrance into the abdomen is obtained in the left upper quadrant with either veress insufflation and 12mm optical trocar placement or open technique with subsequent placement of a 12 mm trocar and insufflation of the abdomen to 15 mm Hg. A 10 mm 30-degree scope is then placed in the left upper quadrant and once safe entry is confirmed the abdomen may be visually inspected. A 10 mm supra umbilical port is placed under direct visualization. The three remaining ports range with surgeon preference from 5-12 mm ports and are placed; two in the right upper quadrant and one in the left upper quadrant usually laterally to the first trocar. The primary surgeon will utilize the two right upper quadrant ports and the assistant will use the two left upper quadrant ports. Another assistant may be required to operate the camera at the umbilical port. if the first assistant is using both left-sided trocars.
- Creation of the roux-limb: Now the greater omentum is elevated along with the transverse colon to expose the ligament of treitz. The jejunum is then divided with an endoscopic stapling device approximately 40 to 50 cm distal to the duodenal-jejunal flexure. The mesentery of the small bowel is divided with either an energy sealing device or a stapling device to gain length of the Roux limb (distal jejunal limb.) Some may mark this roux limb with a suture or Penrose drain to avoid confusion later in the surgery.
- Jejunojejunal anastomosis: Next measure 75 to 150 cm distal to the divided bowel and perform a jejunojejunostomy is a side to side fashion. The roux limb length will generally be longer for patients with more severe obesity (BMI>50.) This may be accomplished with a stapler or be done with a laparoscopic suturing technique. The staple technique is accomplished by aligning the antimesenteric borders of the two small bowel segments with a suture. Two enterotomies are made on the antimesenteric borders with an energy device. A side-to-side isoperistaltic jejunojejunostomy is then performed with an endoscopic linear stapler. The remaining enterotomy from the proximal end of the stapler is then closed transversely with the linear stapler. The mesenteric defect is closed with a running nonabsorbable suture.
- Creation of the gastric pouch: Now the patient will be placed in steep reverse Trendelenburg position to facilitate the creation of the gastric pouch. The orogastric tube is removed and a liver retractor is placed in the subxiphoid position with a stab incision (does not require a trocar.) The angle of His is bluntly divided and the gastrohepatic ligament is divided with an endoscopic stapling device. Next, the gastric pouch is ready to be created. The optimal staple height for the stomach should be 3.8-4.1mm. A bougie should be placed by anesthesia. The first staple firing should be horizontal across the stomach at 1-4 cm distal to the GE junction (no more than 5cm distal to GEJ.) Subsequent staple firing is then oriented vertically toward the angle of His along the inserted bougie. This should create a proximal gastric pouch completely remove from the distal alimentary tract and should be approximately 15 to 30 cc.
- Gastrojejunal anastomosis: Roux limb can be brought to the gastric pouch by many methods, the following are the two main techniques:
- Retrocolic retrogastric: This requires creating a defect in the transverse mesocolon and bringing the Roux limb posterior to the remnant stomach. This creates a third potential space for the development of an internal hernia and must be closed prior to completion of the procedure.
- Antecolic, antegastric: This brings the roux limb anterior to the transverse colon and anterior to the remnant stomach. With both techniques, the space between the roux limb mesentery and the transverse mesocolon must be closed with suture (Petersen space) in addition to the space created by the mesenteries of the jejunojejunal anastomosis.
The anastomosis generally can be performed with three different techniques:
- Circular stapling anastomosis (typically with a 25 mm circular stapler) requires the introduction of an anvil into the stomach pouch via endoscopic or transgastric means. Then the stapler is then introduced into the jejunum through an enterotomy. The anvil is connected to the stapler, and it is fired
- Handsewn anastomosis in a single or two layer running fashion.
- Linear stapling anastomosis (typically with a 45 or 60 mm blue load) creating a side to side gastrojejunal anastomosis. The residual defect where the stapler resided will be closed with laparoscopic sutures.
- Endoscopy: An endoscope may be passed into the pouch to visualize the anastomosis for bleeding and patency. The Roux limb is clamped just distal to the gastrojejunostomy and is submerged in saline. The gastric pouch is then insufflated and is monitored for air bubbles. If a leak is found reinforcement with sutures.
- Closure: All port sites 10mm or larger require fascial closure and the skin is closed with absorbable subcuticular 4-0 sutures.
The evidence behind the use of the different techniques includes the following:
- Roux limb [7]
- Should be at least 75 cm as bile reflux has been seen with roux limbs of 60 cm or less
- Ideally, between 75 to 150 cm, no differences in outcomes have been consistently reported when considering different roux limb length in patients with a BMI less than 50
- There may be clinical use in a longer roux limb (greater than 150 cm) in patients who have a BMI greater than 50.
- The gastric pouch
- Must be a vertical pouch excluding the distensible funds which may lead to increased capacity and less weight loss
- Volume should be between 15 to 30 mL, and no differences in outcomes between the two volumes have been noted
- The pouch must be completely divided from the remnant to prevent a gastro-gastric fistula.
- Roux limb position [8]
- Antecolic passes anterior to transverse colon and does not require a creation of a defect in the transverse mesocolon which is a potential site for an internal hernia. It is associated with increased tension due to the longer length required to reach the stomach and may be associated with increased GJ leaks due to tension on the anastomosis.
- Retrocolic roux limb requires creating a defect in the transverse mesocolon. This creates three potential spaces for an internal hernia: (1) a defect in the mesentery between the enteroenterostomy site, (2) the Petersen defect, which is the space between the roux limb mesentery and the transverse mesocolon, and finally, (3) the defect created in the transverse
- Mesocolon required for a retrocolic roux limb technique is at increased risk of postoperative internal hernias
- Gastrojejunal anastomosis
- There is controversy on whether the technique of GJ anastomosis contributes to postoperative complications. There is evidence to support equal outcomes for all three techniques when considering postoperative leak, stricture, and marginal ulcer formation. [9]
- There is some evidence to support hand-sewn anastomosis over linear and circular stapled anastomosis. Hand sewed GJ anastomosis has been shown to have lower leak and stricture rates when compared to the other two techniques. it may be more technically challenging and increase operative time. [10]
- Circular stapling can be done transorally, which may lead to injury of the upper GI tract, or transgastric which requires extraction of the anvil from the abdomen leading to higher wound infection rate.[11]
Complications
Ninety-day mortality is very low (less than 0.5%). Morbidity of the procedure is classified into early complications and late complications. [4]
Early Complications (0-30 days)
- VTE
- Anastomotic leak
- Infection
- Intestinal obstruction
- GJ stenosis
Late Complications
- Intestinal obstruction
- Dumping syndrome
- Marginal ulcer
- Gastrogastric fistula
- Gallstones
- Incisional hernia
- Nutritional deficiencies
Further information on complications after gastrectomy is out of the scope of this review article. These are very complex disease processes with specific etiologies, pathophysiologies, and treatments.
Clinical Significance
The laparoscopic gastric bypass is currently the second most popular weight loss surgery in the United States of America. It has clear benefits over the traditional open approach in terms of cost and patient satisfaction with similar successes. Patients can expect to have approximately 60% excess weight loss and an exceptional long-term resolution of obesity-related comorbidities after laparoscopic gastric bypass. Although there is a steep learning curve, once one has become an experienced surgeon, a laparoscopic gastric bypass can be performed with low morbidity and excellent outcomes.
Enhancing Healthcare Team Outcomes
Management of obesity is complex and is best done with an interprofessional team that includes the primary care provider, nurse practitioner, dietitian, and surgeon. While there are several bariatric procedures to reverse obesity, all are fraught with serious complications. The primary care providers should educate the patients on changes in lifestyle like a healthy diet and regular exercise. The other fact is to inform the patient to be realistic- non-surgical methods of weight loss do work but they take time. Anyone undergoing a bariatric procedure should be informed about all the potential complications of surgery and the need to remain compliant with the new lifestyle changes, or risk putting the weight back on.[12]