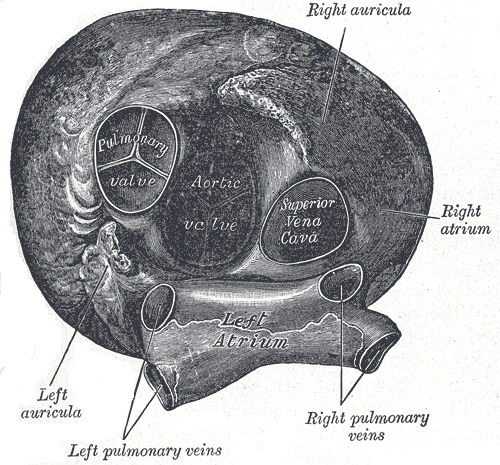
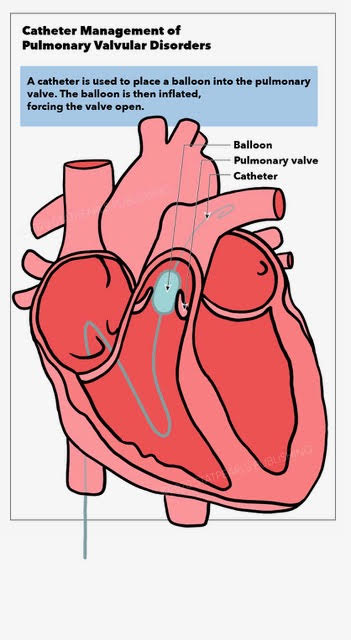
[1]
Marelli AJ,Mackie AS,Ionescu-Ittu R,Rahme E,Pilote L, Congenital heart disease in the general population: changing prevalence and age distribution. Circulation. 2007 Jan 16;
[PubMed PMID: 17210844]
Level 3 (low-level) evidence
[2]
Warnes CA,Liberthson R,Danielson GK,Dore A,Harris L,Hoffman JI,Somerville J,Williams RG,Webb GD, Task force 1: the changing profile of congenital heart disease in adult life. Journal of the American College of Cardiology. 2001 Apr;
[PubMed PMID: 11300418]
[3]
Verheugt CL,Uiterwaal CS,van der Velde ET,Meijboom FJ,Pieper PG,van Dijk AP,Vliegen HW,Grobbee DE,Mulder BJ, Mortality in adult congenital heart disease. European heart journal. 2010 May;
[PubMed PMID: 20207625]
[4]
Yuan SM,Mishaly D,Shinfeld A,Raanani E, Right ventricular outflow tract reconstruction: valved conduit of choice and clinical outcomes. Journal of cardiovascular medicine (Hagerstown, Md.). 2008 Apr;
[PubMed PMID: 18334887]
Level 2 (mid-level) evidence
[5]
Ong K,Boone R,Gao M,Carere R,Webb J,Kiess M,Grewal J, Right ventricle to pulmonary artery conduit reoperations in patients with tetralogy of fallot or pulmonary atresia associated with ventricular septal defect. The American journal of cardiology. 2013 Jun 1;
[PubMed PMID: 23481618]
[6]
Boethig D,Thies WR,Hecker H,Breymann T, Mid term course after pediatric right ventricular outflow tract reconstruction: a comparison of homografts, porcine xenografts and Contegras. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005 Jan;
[PubMed PMID: 15621472]
[7]
Elahi M,Dhannapuneni R,Firmin R,Hickey M, Direct complications of repeat median sternotomy in adults. Asian cardiovascular
[PubMed PMID: 15905341]
[8]
Temeck BK,Katz NM,Wallace RB, An approach to reoperative median sternotomy. Journal of cardiac surgery. 1990 Mar;
[PubMed PMID: 2133819]
[9]
van der Bom T,Zomer AC,Zwinderman AH,Meijboom FJ,Bouma BJ,Mulder BJ, The changing epidemiology of congenital heart disease. Nature reviews. Cardiology. 2011 Jan;
[PubMed PMID: 21045784]
[10]
Vida VL,Berggren H,Brawn WJ,Daenen W,Di Carlo D,Di Donato R,Lindberg HL,Corno AF,Fragata J,Elliott MJ,Hraska V,Kiraly L,Lacour-Gayet F,Maruszewski B,Rubay J,Sairanen H,Sarris G,Urban A,Van Doorn C,Ziemer G,Stellin G, Risk of surgery for congenital heart disease in the adult: a multicentered European study. The Annals of thoracic surgery. 2007 Jan;
[PubMed PMID: 17184653]
[11]
Verheugt CL,Uiterwaal CS,Grobbee DE,Mulder BJ, Long-term prognosis of congenital heart defects: a systematic review. International journal of cardiology. 2008 Dec 17;
[PubMed PMID: 18687485]
Level 1 (high-level) evidence
[12]
Khairy P, Aboulhosn J, Gurvitz MZ, Opotowsky AR, Mongeon FP, Kay J, Valente AM, Earing MG, Lui G, Gersony DR, Cook S, Ting JG, Nickolaus MJ, Webb G, Landzberg MJ, Broberg CS, Alliance for Adult Research in Congenital Cardiology (AARCC). Arrhythmia burden in adults with surgically repaired tetralogy of Fallot: a multi-institutional study. Circulation. 2010 Aug 31:122(9):868-75. doi: 10.1161/CIRCULATIONAHA.109.928481. Epub 2010 Aug 16
[PubMed PMID: 20713900]
[13]
Bonhoeffer P,Boudjemline Y,Saliba Z,Merckx J,Aggoun Y,Bonnet D,Acar P,Le Bidois J,Sidi D,Kachaner J, Percutaneous replacement of pulmonary valve in a right-ventricle to pulmonary-artery prosthetic conduit with valve dysfunction. Lancet (London, England). 2000 Oct 21;
[PubMed PMID: 11052583]
[14]
Peng LF,McElhinney DB,Nugent AW,Powell AJ,Marshall AC,Bacha EA,Lock JE, Endovascular stenting of obstructed right ventricle-to-pulmonary artery conduits: a 15-year experience. Circulation. 2006 Jun 6;
[PubMed PMID: 16735676]
[15]
Putman LM,van Gameren M,Meijboom FJ,de Jong PL,Roos-Hesselink JW,Witsenburg M,Takkenberg JJ,Bogers AJ, Seventeen years of adult congenital heart surgery: a single centre experience. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2009 Jul;
[PubMed PMID: 19303791]
[16]
Coats L,Tsang V,Khambadkone S,van Doorn C,Cullen S,Deanfield J,de Leval MR,Bonhoeffer P, The potential impact of percutaneous pulmonary valve stent implantation on right ventricular outflow tract re-intervention. European journal of cardio-thoracic surgery : official journal of the European Association for Cardio-thoracic Surgery. 2005 Apr;
[PubMed PMID: 15784347]
[17]
Berman DP,McElhinney DB,Vincent JA,Hellenbrand WE,Zahn EM, Feasibility and short-term outcomes of percutaneous transcatheter pulmonary valve replacement in small (<30 kg) children with dysfunctional right ventricular outflow tract conduits. Circulation. Cardiovascular interventions. 2014 Apr;
[PubMed PMID: 24569596]
Level 2 (mid-level) evidence
[18]
Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of Cardiology (ESC), Association for European Paediatric Cardiology (AEPC), ESC Committee for Practice Guidelines (CPG). ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). European heart journal. 2010 Dec:31(23):2915-57. doi: 10.1093/eurheartj/ehq249. Epub 2010 Aug 27
[PubMed PMID: 20801927]
Level 1 (high-level) evidence
[19]
Feltes TF,Bacha E,Beekman RH 3rd,Cheatham JP,Feinstein JA,Gomes AS,Hijazi ZM,Ing FF,de Moor M,Morrow WR,Mullins CE,Taubert KA,Zahn EM, Indications for cardiac catheterization and intervention in pediatric cardiac disease: a scientific statement from the American Heart Association. Circulation. 2011 Jun 7;
[PubMed PMID: 21536996]
[20]
Geva T. Repaired tetralogy of Fallot: the roles of cardiovascular magnetic resonance in evaluating pathophysiology and for pulmonary valve replacement decision support. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2011 Jan 20:13(1):9. doi: 10.1186/1532-429X-13-9. Epub 2011 Jan 20
[PubMed PMID: 21251297]
[21]
Silversides CK,Marelli A,Beauchesne L,Dore A,Kiess M,Salehian O,Bradley T,Colman J,Connelly M,Harris L,Khairy P,Mital S,Niwa K,Oechslin E,Poirier N,Schwerzmann M,Taylor D,Vonder Muhll I,Baumgartner H,Benson L,Celermajer D,Greutmann M,Horlick E,Landzberg M,Meijboom F,Mulder B,Warnes C,Webb G,Therrien J, Canadian Cardiovascular Society 2009 Consensus Conference on the management of adults with congenital heart disease: executive summary. The Canadian journal of cardiology. 2010 Mar;
[PubMed PMID: 20352134]
Level 3 (low-level) evidence
[22]
Warnes CA,Williams RG,Bashore TM,Child JS,Connolly HM,Dearani JA,Del Nido P,Fasules JW,Graham TP Jr,Hijazi ZM,Hunt SA,King ME,Landzberg MJ,Miner PD,Radford MJ,Walsh EP,Webb GD, ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Journal of the American College of Cardiology. 2008 Dec 2;
[PubMed PMID: 19038677]
Level 1 (high-level) evidence
[23]
Andersen HR,Knudsen LL,Hasenkam JM, Transluminal implantation of artificial heart valves. Description of a new expandable aortic valve and initial results with implantation by catheter technique in closed chest pigs. European heart journal. 1992 May;
[PubMed PMID: 1618213]
[24]
Mulder BJ,de Winter RJ,Wilde AA, Percutaneous pulmonary valve replacement: a new development in the lifetime strategy for patients with congenital heart disease. Netherlands heart journal : monthly journal of the Netherlands Society of Cardiology and the Netherlands Heart Foundation. 2007 Jan;
[PubMed PMID: 17612700]
[25]
Schievano S,Coats L,Migliavacca F,Norman W,Frigiola A,Deanfield J,Bonhoeffer P,Taylor AM, Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2007;
[PubMed PMID: 17578725]
[26]
Kheiwa A,Divanji P,Mahadevan VS, Transcatheter pulmonary valve implantation: will it replace surgical pulmonary valve replacement? Expert review of cardiovascular therapy. 2018 Mar;
[PubMed PMID: 29433351]
[28]
Gillespie MJ,Dori Y,Harris MA,Sathanandam S,Glatz AC,Rome JJ, Bilateral branch pulmonary artery melody valve implantation for treatment of complex right ventricular outflow tract dysfunction in a high-risk patient. Circulation. Cardiovascular interventions. 2011 Aug;
[PubMed PMID: 21846891]
[29]
Chung R,Taylor AM, Imaging for preintervention planning: transcatheter pulmonary valve therapy. Circulation. Cardiovascular imaging. 2014 Jan;
[PubMed PMID: 24449547]
[30]
Hascoët S,Acar P,Boudjemline Y, Transcatheter pulmonary valvulation: current indications and available devices. Archives of cardiovascular diseases. 2014 Nov;
[PubMed PMID: 25444020]
[31]
Boudjemline Y,Sarquella-Brugada G,Kamache I,Patel M,Ladouceur M,Bonnet D,Boughenou FM,Fraisse A,Iserin L, Impact of right ventricular outflow tract size and substrate on outcomes of percutaneous pulmonary valve implantation. Archives of cardiovascular diseases. 2013 Jan;
[PubMed PMID: 23374968]
[32]
Alsulami G,Patel M,Malekzadeh-Milani S,Bonnet D,Boudjemline Y, Hyperacute flash pulmonary oedema after transcatheter pulmonary valve implantation: The melody of an overwhelmed left ventricle. Archives of cardiovascular diseases. 2014 Apr;
[PubMed PMID: 24793996]
[33]
Khambadkone S,Coats L,Taylor A,Boudjemline Y,Derrick G,Tsang V,Cooper J,Muthurangu V,Hegde SR,Razavi RS,Pellerin D,Deanfield J,Bonhoeffer P, Percutaneous pulmonary valve implantation in humans: results in 59 consecutive patients. Circulation. 2005 Aug 23;
[PubMed PMID: 16103239]
[34]
Schievano S,Petrini L,Migliavacca F,Coats L,Nordmeyer J,Lurz P,Khambadkone S,Taylor AM,Dubini G,Bonhoeffer P, Finite element analysis of stent deployment: understanding stent fracture in percutaneous pulmonary valve implantation. Journal of interventional cardiology. 2007 Dec;
[PubMed PMID: 18042059]
Level 3 (low-level) evidence
[35]
Gillespie MJ,Rome JJ,Levi DS,Williams RJ,Rhodes JF,Cheatham JP,Hellenbrand WE,Jones TK,Vincent JA,Zahn EM,McElhinney DB, Melody valve implant within failed bioprosthetic valves in the pulmonary position: a multicenter experience. Circulation. Cardiovascular interventions. 2012 Dec;
[PubMed PMID: 23212395]
[36]
Boshoff DE,Cools BL,Heying R,Troost E,Kefer J,Budts W,Gewillig M, Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography
[PubMed PMID: 22887796]
[37]
Meadows JJ,Moore PM,Berman DP,Cheatham JP,Cheatham SL,Porras D,Gillespie MJ,Rome JJ,Zahn EM,McElhinney DB, Use and performance of the Melody Transcatheter Pulmonary Valve in native and postsurgical, nonconduit right ventricular outflow tracts. Circulation. Cardiovascular interventions. 2014 Jun;
[PubMed PMID: 24867892]
[38]
Cao QL,Kenny D,Zhou D,Pan W,Guan L,Ge J,Hijazi ZM, Early clinical experience with a novel self-expanding percutaneous stent-valve in the native right ventricular outflow tract. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography
[PubMed PMID: 24824357]
[39]
Alkashkari W,Alsubei A,Hijazi ZM, Transcatheter Pulmonary Valve Replacement: Current State of Art. Current cardiology reports. 2018 Mar 15;
[PubMed PMID: 29546472]
[40]
Morray BH,McElhinney DB,Cheatham JP,Zahn EM,Berman DP,Sullivan PM,Lock JE,Jones TK, Risk of coronary artery compression among patients referred for transcatheter pulmonary valve implantation: a multicenter experience. Circulation. Cardiovascular interventions. 2013 Oct 1;
[PubMed PMID: 24065444]
[41]
Shah N,Cheng VE,Cox N,Soon K, Percutaneous Coronary Intervention of an Anomalous Left Main Coronary Artery Arising from the Right Sinus of Valsalva. Heart, lung
[PubMed PMID: 25911146]
[42]
Sridharan S,Coats L,Khambadkone S,Taylor AM,Bonhoeffer P, Images in cardiovascular medicine. Transcatheter right ventricular outflow tract intervention: the risk to the coronary circulation. Circulation. 2006 Jun 27;
[PubMed PMID: 16801469]
[43]
Lindsay I,Aboulhosn J,Salem M,Levi D, Aortic root compression during transcatheter pulmonary valve replacement. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography
[PubMed PMID: 27121036]
[44]
Biernacka EK,Rużyłło W,Demkow M,Kowalski M,Śpiewak M,Piotrowski W,Kuśmierczyk M,Banaś S,Różanski J,Hoffman P, Transcatheter pulmonary valve implantation in patients with right ventricular outflow tract dysfunction: early and mid-term results. The Journal of invasive cardiology. 2015 Jun;
[PubMed PMID: 26028663]
[45]
Boudjemline Y,Malekzadeh-Milani S,Patel M,Thambo JB,Bonnet D,Iserin L,Fraisse A, Predictors and outcomes of right ventricular outflow tract conduit rupture during percutaneous pulmonary valve implantation: a multicentre study. EuroIntervention : journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2016 Jan 22;
[PubMed PMID: 25244126]
[46]
McElhinney DB,Cheatham JP,Jones TK,Lock JE,Vincent JA,Zahn EM,Hellenbrand WE, Stent fracture, valve dysfunction, and right ventricular outflow tract reintervention after transcatheter pulmonary valve implantation: patient-related and procedural risk factors in the US Melody Valve Trial. Circulation. Cardiovascular interventions. 2011 Dec 1;
[PubMed PMID: 22075927]
[47]
McElhinney DB,Bergersen L,Marshall AC, In situ fracture of stents implanted for relief of pulmonary arterial stenosis in patients with congenitally malformed hearts. Cardiology in the young. 2008 Aug;
[PubMed PMID: 18559137]
[48]
Cheung G,Vejlstrup N,Ihlemann N,Arnous S,Franzen O,Bundgaard H,Søndergaard L, Infective endocarditis following percutaneous pulmonary valve replacement: diagnostic challenges and application of intra-cardiac echocardiography. International journal of cardiology. 2013 Nov 30;
[PubMed PMID: 24182680]
[49]
Lurz P,Coats L,Khambadkone S,Nordmeyer J,Boudjemline Y,Schievano S,Muthurangu V,Lee TY,Parenzan G,Derrick G,Cullen S,Walker F,Tsang V,Deanfield J,Taylor AM,Bonhoeffer P, Percutaneous pulmonary valve implantation: impact of evolving technology and learning curve on clinical outcome. Circulation. 2008 Apr 15;
[PubMed PMID: 18391109]
Level 2 (mid-level) evidence
[50]
Chen XJ,Smith PB,Jaggers J,Lodge AJ, Bioprosthetic pulmonary valve replacement: contemporary analysis of a large, single-center series of 170 cases. The Journal of thoracic and cardiovascular surgery. 2013 Dec;
[PubMed PMID: 23122698]
Level 3 (low-level) evidence
[51]
Buber J,Bergersen L,Lock JE,Gauvreau K,Esch JJ,Landzberg MJ,Valente AM,Sandora TJ,Marshall AC, Bloodstream infections occurring in patients with percutaneously implanted bioprosthetic pulmonary valve: a single-center experience. Circulation. Cardiovascular interventions. 2013 Jun;
[PubMed PMID: 23756696]
[52]
Patel M,Malekzadeh-Milani S,Ladouceur M,Iserin L,Boudjemline Y, Percutaneous pulmonary valve endocarditis: incidence, prevention and management. Archives of cardiovascular diseases. 2014 Nov;
[PubMed PMID: 25445753]
[53]
Cheatham JP,Hellenbrand WE,Zahn EM,Jones TK,Berman DP,Vincent JA,McElhinney DB, Clinical and hemodynamic outcomes up to 7 years after transcatheter pulmonary valve replacement in the US melody valve investigational device exemption trial. Circulation. 2015 Jun 2;
[PubMed PMID: 25944758]
[54]
Vezmar M,Chaturvedi R,Lee KJ,Almeida C,Manlhiot C,McCrindle BW,Horlick EM,Benson LN, Percutaneous pulmonary valve implantation in the young 2-year follow-up. JACC. Cardiovascular interventions. 2010 Apr;
[PubMed PMID: 20398873]
[55]
Asoh K,Walsh M,Hickey E,Nagiub M,Chaturvedi R,Lee KJ,Benson LN, Percutaneous pulmonary valve implantation within bioprosthetic valves. European heart journal. 2010 Jun;
[PubMed PMID: 20231157]
[56]
Demkow M,Biernacka EK,Spiewak M,Kowalski M,Siudalska H,Wolski P,Sondergaard L,Miśko J,Hoffman P,Rużyłło W, Percutaneous pulmonary valve implantation preceded by routine prestenting with a bare metal stent. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography
[PubMed PMID: 20602475]
[57]
Coats L,Khambadkone S,Derrick G,Hughes M,Jones R,Mist B,Pellerin D,Marek J,Deanfield JE,Bonhoeffer P,Taylor AM, Physiological consequences of percutaneous pulmonary valve implantation: the different behaviour of volume- and pressure-overloaded ventricles. European heart journal. 2007 Aug;
[PubMed PMID: 17595193]
[58]
Zahn EM,Hellenbrand WE,Lock JE,McElhinney DB, Implantation of the melody transcatheter pulmonary valve in patients with a dysfunctional right ventricular outflow tract conduit early results from the u.s. Clinical trial. Journal of the American College of Cardiology. 2009 Oct 27;
[PubMed PMID: 19850214]
[59]
Martins JD,Ewert P,Sousa L,Freitas I,Trigo C,Jalles N,Matos P,Agapito A,Ferreira R,Pinto FF, Percutaneous pulmonary valve implantation: initial experience. Revista portuguesa de cardiologia : orgao oficial da Sociedade Portuguesa de Cardiologia = Portuguese journal of cardiology : an official journal of the Portuguese Society of Cardiology. 2010 Dec;
[PubMed PMID: 21428139]
[60]
Batra AS,McElhinney DB,Wang W,Zakheim R,Garofano RP,Daniels C,Yung D,Cooper DM,Rhodes J, Cardiopulmonary exercise function among patients undergoing transcatheter pulmonary valve implantation in the US Melody valve investigational trial. American heart journal. 2012 Feb;
[PubMed PMID: 22305848]
[61]
Borik S,Crean A,Horlick E,Osten M,Lee KJ,Chaturvedi R,Friedberg MK,McCrindle BW,Manlhiot C,Benson L, Percutaneous pulmonary valve implantation: 5 years of follow-up: does age influence outcomes? Circulation. Cardiovascular interventions. 2015 Feb;
[PubMed PMID: 25652317]
[62]
Holzer RJ,Hijazi ZM, Transcatheter pulmonary valve replacement: State of the art. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography
[PubMed PMID: 26423185]
[63]
Armstrong AK,Balzer DT,Cabalka AK,Gray RG,Javois AJ,Moore JW,Rome JJ,Turner DR,Zellers TM,Kreutzer J, One-year follow-up of the Melody transcatheter pulmonary valve multicenter post-approval study. JACC. Cardiovascular interventions. 2014 Nov;
[PubMed PMID: 25459038]
[64]
Kenny D,Hijazi ZM,Kar S,Rhodes J,Mullen M,Makkar R,Shirali G,Fogel M,Fahey J,Heitschmidt MG,Cain C, Percutaneous implantation of the Edwards SAPIEN transcatheter heart valve for conduit failure in the pulmonary position: early phase 1 results from an international multicenter clinical trial. Journal of the American College of Cardiology. 2011 Nov 15;
[PubMed PMID: 22078433]
Level 1 (high-level) evidence
[65]
Faza N,Kenny D,Kavinsky C,Amin Z,Heitschmidt M,Hijazi ZM, Single-center comparative outcomes of the Edwards SAPIEN and Medtronic Melody transcatheter heart valves in the pulmonary position. Catheterization and cardiovascular interventions : official journal of the Society for Cardiac Angiography
[PubMed PMID: 23008193]
Level 2 (mid-level) evidence