[1]
Abrey LE,Ben-Porat L,Panageas KS,Yahalom J,Berkey B,Curran W,Schultz C,Leibel S,Nelson D,Mehta M,DeAngelis LM, Primary central nervous system lymphoma: the Memorial Sloan-Kettering Cancer Center prognostic model. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2006 Dec 20;
[PubMed PMID: 17116938]
[2]
COOPER EL,RIKER JL, Malignant lymphoma of the uveal tract. American journal of ophthalmology. 1951 Aug;
[PubMed PMID: 14857107]
[4]
Mulay K,Narula R,Honavar SG, Primary vitreoretinal lymphoma. Indian journal of ophthalmology. 2015 Mar;
[PubMed PMID: 25971162]
[5]
Raval V,Binkley E,Aronow ME,Valenzuela J,Peereboom DM,Singh AD, Primary central nervous system lymphoma - ocular variant: an interdisciplinary review on management. Survey of ophthalmology. 2021 Nov-Dec;
[PubMed PMID: 33762182]
Level 3 (low-level) evidence
[6]
Buggage RR,Chan CC,Nussenblatt RB, Ocular manifestations of central nervous system lymphoma. Current opinion in oncology. 2001 May;
[PubMed PMID: 11307054]
Level 3 (low-level) evidence
[7]
Chan CC,Buggage RR,Nussenblatt RB, Intraocular lymphoma. Current opinion in ophthalmology. 2002 Dec;
[PubMed PMID: 12441846]
Level 3 (low-level) evidence
[8]
Coupland SE,Foss HD,Hidayat AA,Cockerham GC,Hummel M,Stein H, Extranodal marginal zone B cell lymphomas of the uvea: an analysis of 13 cases. The Journal of pathology. 2002 Jul;
[PubMed PMID: 12115879]
Level 3 (low-level) evidence
[9]
Coupland SE,Joussen A,Anastassiou G,Stein H, Diagnosis of a primary uveal extranodal marginal zone B-cell lymphoma by chorioretinal biopsy: case report. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2005 May;
[PubMed PMID: 15586289]
Level 3 (low-level) evidence
[10]
Chan CC,Shen D,Hackett JJ,Buggage RR,Tuaillon N, Expression of chemokine receptors, CXCR4 and CXCR5, and chemokines, BLC and SDF-1, in the eyes of patients with primary intraocular lymphoma. Ophthalmology. 2003 Feb;
[PubMed PMID: 12578791]
[11]
Shannon-Lowe C,Rickinson AB,Bell AI, Epstein-Barr virus-associated lymphomas. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2017 Oct 19
[PubMed PMID: 28893938]
[12]
Ongkosuwito JV,Van der Lelij A,Bruinenberg M,Wienesen-van Doorn M,Feron EJ,Hoyng CB,de Keizer RJ,Klok AM,Kijlstra A, Increased presence of Epstein-Barr virus DNA in ocular fluid samples from HIV negative immunocompromised patients with uveitis. The British journal of ophthalmology. 1998 Mar;
[PubMed PMID: 9602620]
[13]
Shen DF,Herbort CP,Tuaillon N,Buggage RR,Egwuagu CE,Chan CC, Detection of Toxoplasma gondii DNA in primary intraocular B-cell lymphoma. Modern pathology : an official journal of the United States and Canadian Academy of Pathology, Inc. 2001 Oct;
[PubMed PMID: 11598169]
[14]
Ahluwalia MS,Peereboom DM, Primary central nervous system lymphoma. Current treatment options in neurology. 2010 Jul;
[PubMed PMID: 20842593]
[15]
Rivero ME,Kuppermann BD,Wiley CA,Garcia CR,Smith MD,Dreilinger A,Freeman WR, Acquired immunodeficiency syndrome-related intraocular B-cell lymphoma. Archives of ophthalmology (Chicago, Ill. : 1960). 1999 May;
[PubMed PMID: 10326958]
[16]
Goldberg DE,Smithen LM,Angelilli A,Freeman WR, HIV-associated retinopathy in the HAART era. Retina (Philadelphia, Pa.). 2005 Jul-Aug;
[PubMed PMID: 16077362]
[17]
Cassoux N,Merle-Beral H,Leblond V,Bodaghi B,Miléa D,Gerber S,Fardeau C,Reux I,Xuan KH,Chan CC,LeHoang P, Ocular and central nervous system lymphoma: clinical features and diagnosis. Ocular immunology and inflammation. 2000 Dec;
[PubMed PMID: 11262654]
[18]
Rajagopal R,Harbour JW, Diagnostic testing and treatment choices in primary vitreoretinal lymphoma. Retina (Philadelphia, Pa.). 2011 Mar;
[PubMed PMID: 21336066]
[19]
Berenbom A,Davila RM,Lin HS,Harbour JW, Treatment outcomes for primary intraocular lymphoma: implications for external beam radiotherapy. Eye (London, England). 2007 Sep;
[PubMed PMID: 16732210]
[20]
Char DH,Ljung BM,Deschênes J,Miller TR, Intraocular lymphoma: immunological and cytological analysis. The British journal of ophthalmology. 1988 Dec;
[PubMed PMID: 3067746]
[21]
Bhat PV,Jakobiec FA,Papaliodis G,Sobrin L, Primary T-cell lymphoma of the retina and cerebellum: immunophenotypic and gene rearrangement confirmation. American journal of ophthalmology. 2009 Sep;
[PubMed PMID: 19477711]
[22]
Coupland SE,Loddenkemper C,Smith JR,Braziel RM,Charlotte F,Anagnostopoulos I,Stein H, Expression of immunoglobulin transcription factors in primary intraocular lymphoma and primary central nervous system lymphoma. Investigative ophthalmology
[PubMed PMID: 16249468]
[23]
Grimm SA,Pulido JS,Jahnke K,Schiff D,Hall AJ,Shenkier TN,Siegal T,Doolittle ND,Batchelor T,Herrlinger U,Neuwelt EA,Laperriere N,Chamberlain MC,Blay JY,Ferreri AJ,Omuro AM,Thiel E,Abrey LE, Primary intraocular lymphoma: an International Primary Central Nervous System Lymphoma Collaborative Group Report. Annals of oncology : official journal of the European Society for Medical Oncology. 2007 Nov;
[PubMed PMID: 17804469]
[25]
Sagoo MS,Mehta H,Swampillai AJ,Cohen VM,Amin SZ,Plowman PN,Lightman S, Primary intraocular lymphoma. Survey of ophthalmology. 2014 Sep-Oct;
[PubMed PMID: 24560125]
Level 3 (low-level) evidence
[26]
Akpek EK,Ahmed I,Hochberg FH,Soheilian M,Dryja TP,Jakobiec FA,Foster CS, Intraocular-central nervous system lymphoma: clinical features, diagnosis, and outcomes. Ophthalmology. 1999 Sep;
[PubMed PMID: 10485554]
[27]
Hoffman PM,McKelvie P,Hall AJ,Stawell RJ,Santamaria JD, Intraocular lymphoma: a series of 14 patients with clinicopathological features and treatment outcomes. Eye (London, England). 2003 May;
[PubMed PMID: 12802353]
[28]
Chan CC,Rubenstein JL,Coupland SE,Davis JL,Harbour JW,Johnston PB,Cassoux N,Touitou V,Smith JR,Batchelor TT,Pulido JS, Primary vitreoretinal lymphoma: a report from an International Primary Central Nervous System Lymphoma Collaborative Group symposium. The oncologist. 2011;
[PubMed PMID: 22045784]
[29]
Marchese A,Miserocchi E,Giuffrè C,Cicinelli MV,Querques G,Bandello F,Modorati G, Aurora borealis and string of pearls in vitreoretinal lymphoma: patterns of vitreous haze. The British journal of ophthalmology. 2019 Nov;
[PubMed PMID: 30709808]
[30]
Gass JD,Sever RJ,Grizzard WS,Clarkson JG,Blumenkranz M,Wind CA,Shugarman R, Multifocal pigment epithelial detachments by reticulum cell sarcoma. A characteristic funduscopic picture. Retina (Philadelphia, Pa.). 1984 Summer-Fall;
[PubMed PMID: 6387833]
[31]
Dean JM,Novak MA,Chan CC,Green WR, Tumor detachments of the retinal pigment epithelium in ocular/ central nervous system lymphoma. Retina (Philadelphia, Pa.). 1996;
[PubMed PMID: 8927810]
[32]
Fardeau C,Lee CP,Merle-Béral H,Cassoux N,Bodaghi B,Davi F,Lehoang P, Retinal fluorescein, indocyanine green angiography, and optic coherence tomography in non-Hodgkin primary intraocular lymphoma. American journal of ophthalmology. 2009 May;
[PubMed PMID: 19243734]
[33]
Noda S,Hayasaka S,Setogawa T, Intraocular lymphoma invades the optic nerve and orbit. Annals of ophthalmology. 1993 Jan;
[PubMed PMID: 8427488]
[34]
Gill MK,Jampol LM, Variations in the presentation of primary intraocular lymphoma: case reports and a review. Survey of ophthalmology. 2001 May-Jun;
[PubMed PMID: 11425352]
Level 3 (low-level) evidence
[36]
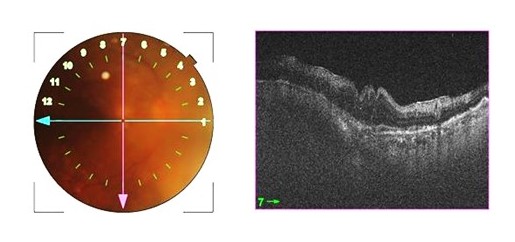
Forooghian F,Merkur AB,White VA,Shen D,Chan CC, High-definition optical coherence tomography features of primary vitreoretinal lymphoma. Ophthalmic surgery, lasers
[PubMed PMID: 21956854]
[37]
Velez G,Chan CC,Csaky KG, Fluorescein angiographic findings in primary intraocular lymphoma. Retina (Philadelphia, Pa.). 2002 Feb;
[PubMed PMID: 11884876]
[38]
Casady M,Faia L,Nazemzadeh M,Nussenblatt R,Chan CC,Sen HN, Fundus autofluorescence patterns in primary intraocular lymphoma. Retina (Philadelphia, Pa.). 2014 Feb;
[PubMed PMID: 23958842]
[39]
Ursea R,Heinemann MH,Silverman RH,Deangelis LM,Daly SW,Coleman DJ, Ophthalmic, ultrasonographic findings in primary central nervous system lymphoma with ocular involvement. Retina (Philadelphia, Pa.). 1997;
[PubMed PMID: 9143039]
[40]
Jack CR Jr,O'Neill BP,Banks PM,Reese DF, Central nervous system lymphoma: histologic types and CT appearance. Radiology. 1988 Apr;
[PubMed PMID: 3279454]
[41]
Bessell EM,Hoang-Xuan K,Ferreri AJ,Reni M, Primary central nervous system lymphoma: biological aspects and controversies in management. European journal of cancer (Oxford, England : 1990). 2007 May;
[PubMed PMID: 17433666]
[42]
Cheng G,Zhang J, Imaging features (CT, MRI, MRS, and PET/CT) of primary central nervous system lymphoma in immunocompetent patients. Neurological sciences : official journal of the Italian Neurological Society and of the Italian Society of Clinical Neurophysiology. 2019 Mar;
[PubMed PMID: 30580380]
[43]
Hwang CS,Yeh S,Bergstrom CS, Diagnostic vitrectomy for primary intraocular lymphoma: when, why, how? International ophthalmology clinics. 2014 Spring;
[PubMed PMID: 24613891]
[44]
Cursiefen C,Holbach LM,Lafaut B,Heimann K,Kirchner T,Naumann GO, Oculocerebral non-Hodgkin's lymphoma with uveal involvement: development of an epibulbar tumor after vitrectomy. Archives of ophthalmology (Chicago, Ill. : 1960). 2000 Oct;
[PubMed PMID: 11030832]
[45]
Coupland SE,Bechrakis NE,Anastassiou G,Foerster AM,Heiligenhaus A,Pleyer U,Hummel M,Stein H, Evaluation of vitrectomy specimens and chorioretinal biopsies in the diagnosis of primary intraocular lymphoma in patients with Masquerade syndrome. Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2003 Oct;
[PubMed PMID: 14605902]
[46]
Trudeau M,Shepherd FA,Blackstein ME,Gospodarowicz M,Fitzpatrick P,Moffatt KP, Intraocular lymphoma: report of three cases and review of the literature. American journal of clinical oncology. 1988 Apr;
[PubMed PMID: 3282423]
Level 3 (low-level) evidence
[47]
Whitcup SM,Chan CC,Buggage RR,Nussenblatt RB,Byrnes GA,Rubin BI, Improving the diagnostic yield of vitrectomy for intraocular lymphoma. Archives of ophthalmology (Chicago, Ill. : 1960). 2000 Mar;
[PubMed PMID: 10721983]
[48]
Coupland SE,Perez-Canto A,Hummel M,Stein H,Heimann H, Assessment of HOPE fixation in vitrectomy specimens in patients with chronic bilateral uveitis (masquerade syndrome). Graefe's archive for clinical and experimental ophthalmology = Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2005 Sep;
[PubMed PMID: 15909161]
[49]
Nasir S,DeAngelis LM, Update on the management of primary CNS lymphoma. Oncology (Williston Park, N.Y.). 2000 Feb;
[PubMed PMID: 10736810]
[50]
Wang Y,Shen D,Wang VM,Sen HN,Chan CC, Molecular biomarkers for the diagnosis of primary vitreoretinal lymphoma. International journal of molecular sciences. 2011;
[PubMed PMID: 22016619]
[51]
Davis JL,Miller DM,Ruiz P, Diagnostic testing of vitrectomy specimens. American journal of ophthalmology. 2005 Nov;
[PubMed PMID: 16310459]
[53]
Chan CC,Wallace DJ, Intraocular lymphoma: update on diagnosis and management. Cancer control : journal of the Moffitt Cancer Center. 2004 Sep-Oct;
[PubMed PMID: 15377987]
[56]
Sen HN,Bodaghi B,Hoang PL,Nussenblatt R, Primary intraocular lymphoma: diagnosis and differential diagnosis. Ocular immunology and inflammation. 2009 May-Jun;
[PubMed PMID: 19585354]
[58]
Chan CC, Molecular pathology of primary intraocular lymphoma. Transactions of the American Ophthalmological Society. 2003;
[PubMed PMID: 14971583]
[60]
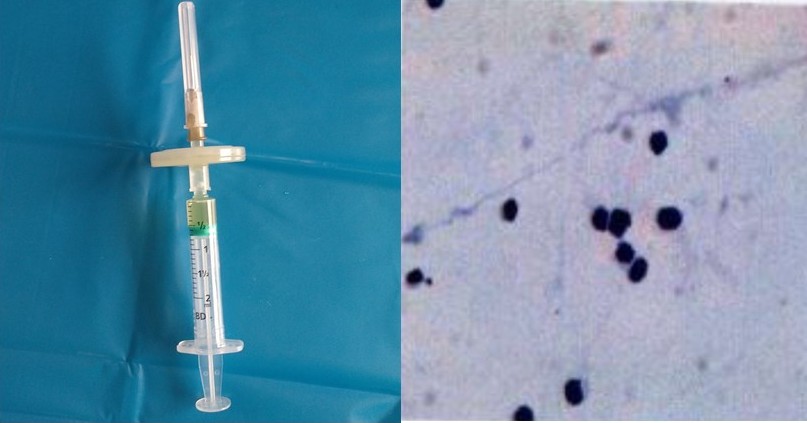
Frenkel S,Hendler K,Siegal T,Shalom E,Pe'er J, Intravitreal methotrexate for treating vitreoretinal lymphoma: 10 years of experience. The British journal of ophthalmology. 2008 Mar;
[PubMed PMID: 18303160]
[61]
de Smet MD,Vancs VS,Kohler D,Solomon D,Chan CC, Intravitreal chemotherapy for the treatment of recurrent intraocular lymphoma. The British journal of ophthalmology. 1999 Apr;
[PubMed PMID: 10434868]
[62]
Jeong Y,Ryu JS,Park UC,Oh JY, Corneal Epithelial Toxicity after Intravitreal Methotrexate Injection for Vitreoretinal Lymphoma: Clinical and In Vitro Studies. Journal of clinical medicine. 2020 Aug 18;
[PubMed PMID: 32824794]
[64]
Larkin KL,Saboo US,Comer GM,Forooghian F,Mackensen F,Merrill P,Sen HN,Singh A,Essex RW,Lake S,Lim LL,Vasconcelos-Santos DV,Foster CS,Wilson DJ,Smith JR, Use of intravitreal rituximab for treatment of vitreoretinal lymphoma. The British journal of ophthalmology. 2014 Jan;
[PubMed PMID: 24158837]
[65]
Hashida N,Ohguro N,Nishida K, Efficacy and Complications of Intravitreal Rituximab Injection for Treating Primary Vitreoretinal Lymphoma. Translational vision science
[PubMed PMID: 24049708]
[66]
Rosenfeld MR,Pruitt AA, Management of malignant gliomas and primary CNS lymphoma: standard of care and future directions. Continuum (Minneapolis, Minn.). 2012 Apr;
[PubMed PMID: 22810135]
Level 3 (low-level) evidence
[68]
DeAngelis LM, Primary CNS lymphoma: treatment with combined chemotherapy and radiotherapy. Journal of neuro-oncology. 1999 Jul;
[PubMed PMID: 10563431]
[69]
Soussain C,Merle-Béral H,Reux I,Sutton L,Fardeau C,Gerber S,Ben Othman T,Binet JL,Lehoang P,Leblond V, A single-center study of 11 patients with intraocular lymphoma treated with conventional chemotherapy followed by high-dose chemotherapy and autologous bone marrow transplantation in 5 cases. Leukemia
[PubMed PMID: 9031115]
Level 3 (low-level) evidence
[70]
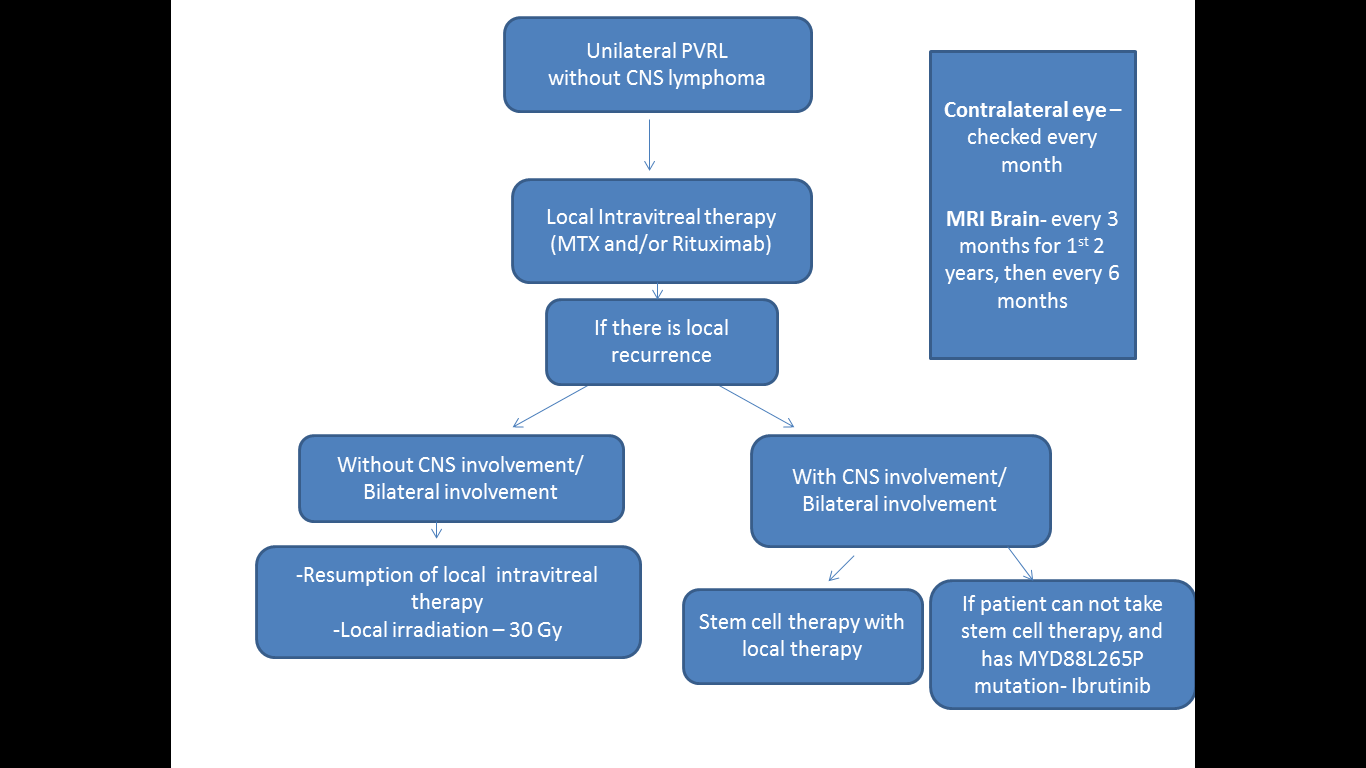
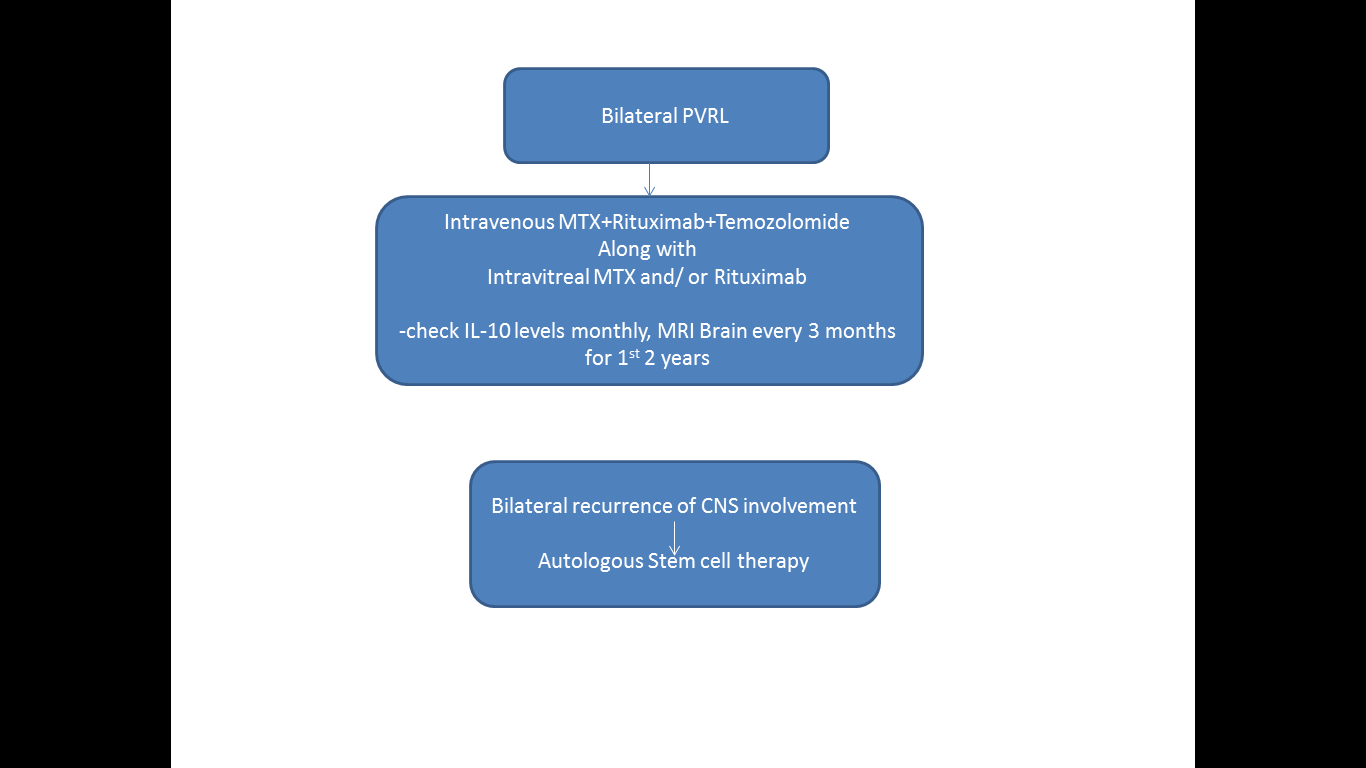
Pulido JS,Johnston PB,Nowakowski GS,Castellino A,Raja H, The diagnosis and treatment of primary vitreoretinal lymphoma: a review. International journal of retina and vitreous. 2018;
[PubMed PMID: 29760948]
[71]
Mason JO,Fischer DH, Intrathecal chemotherapy for recurrent central nervous system intraocular lymphoma. Ophthalmology. 2003 Jun;
[PubMed PMID: 12799254]
[72]
Baumann MA,Ritch PS,Hande KR,Williams GA,Topping TM,Anderson T, Treatment of intraocular lymphoma with high-dose Ara-C. Cancer. 1986 Apr 1;
[PubMed PMID: 2418934]
[73]
Strauchen JA,Dalton J,Friedman AH, Chemotherapy in the management of intraocular lymphoma. Cancer. 1989 May 15
[PubMed PMID: 2702565]
[74]
Valluri S,Moorthy RS,Khan A,Rao NA, Combination treatment of intraocular lymphoma. Retina (Philadelphia, Pa.). 1995;
[PubMed PMID: 7624599]
[75]
Zhang Y,Zhou DB, Primary central nervous system lymphoma: status and advances in diagnosis, molecular pathogenesis, and treatment. Chinese medical journal. 2020 Jun 20;
[PubMed PMID: 32452898]
Level 3 (low-level) evidence
[76]
Soussain C,Hoang-Xuan K,Taillandier L,Fourme E,Choquet S,Witz F,Casasnovas O,Dupriez B,Souleau B,Taksin AL,Gisselbrecht C,Jaccard A,Omuro A,Sanson M,Janvier M,Kolb B,Zini JM,Leblond V,Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire., Intensive chemotherapy followed by hematopoietic stem-cell rescue for refractory and recurrent primary CNS and intraocular lymphoma: Société Française de Greffe de Moëlle Osseuse-Thérapie Cellulaire. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2008 May 20;
[PubMed PMID: 18413641]
[77]
Tun HW,Johnston PB,DeAngelis LM,Atherton PJ,Pederson LD,Koenig PA,Reeder CB,Omuro AMP,Schiff D,O'Neill B,Pulido J,Jaeckle KA,Grommes C,Witzig TE, Phase 1 study of pomalidomide and dexamethasone for relapsed/refractory primary CNS or vitreoretinal lymphoma. Blood. 2018 Nov 22;
[PubMed PMID: 30262659]
[78]
Soussain C,Choquet S,Blonski M,Leclercq D,Houillier C,Rezai K,Bijou F,Houot R,Boyle E,Gressin R,Nicolas-Virelizier E,Barrie M,Moluçon-Chabrot C,Lelez ML,Clavert A,Coisy S,Leruez S,Touitou V,Cassoux N,Daniau M,Ertault de la Bretonnière M,El Yamani A,Ghesquières H,Hoang-Xuan K, Ibrutinib monotherapy for relapse or refractory primary CNS lymphoma and primary vitreoretinal lymphoma: Final analysis of the phase II 'proof-of-concept' iLOC study by the Lymphoma study association (LYSA) and the French oculo-cerebral lymphoma (LOC) network. European journal of cancer (Oxford, England : 1990). 2019 Aug;
[PubMed PMID: 31279304]
[79]
Ahn IE,Jerussi T,Farooqui M,Tian X,Wiestner A,Gea-Banacloche J, Atypical Pneumocystis jirovecii pneumonia in previously untreated patients with CLL on single-agent ibrutinib. Blood. 2016 Oct 13;
[PubMed PMID: 27503501]
[80]
Ghesquieres H,Chevrier M,Laadhari M,Chinot O,Choquet S,Moluçon-Chabrot C,Beauchesne P,Gressin R,Morschhauser F,Schmitt A,Gyan E,Hoang-Xuan K,Nicolas-Virelizier E,Cassoux N,Touitou V,Le Garff-Tavernier M,Savignoni A,Turbiez I,Soumelis V,Houillier C,Soussain C, Lenalidomide in combination with intravenous rituximab (REVRI) in relapsed/refractory primary CNS lymphoma or primary intraocular lymphoma: a multicenter prospective 'proof of concept' phase II study of the French Oculo-Cerebral lymphoma (LOC) Network and the Lymphoma Study Association (LYSA)†. Annals of oncology : official journal of the European Society for Medical Oncology. 2019 Apr 1;
[PubMed PMID: 30698644]
Level 2 (mid-level) evidence
[81]
Plowman PN,Montefiore DS,Lightman S, Multiagent chemotherapy in the salvage cure of ocular lymphoma relapsing after radiotherapy. Clinical oncology (Royal College of Radiologists (Great Britain)). 1993;
[PubMed PMID: 8305342]
[82]
Char DH,Ljung BM,Miller T,Phillips T, Primary intraocular lymphoma (ocular reticulum cell sarcoma) diagnosis and management. Ophthalmology. 1988 May;
[PubMed PMID: 3050698]
[83]
Grommes C,DeAngelis LM, Primary CNS Lymphoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2017 Jul 20;
[PubMed PMID: 28640701]
[84]
Taoka K,Yamamoto G,Kaburaki T,Takahashi T,Araie M,Kurokawa M, Treatment of primary intraocular lymphoma with rituximab, high dose methotrexate, procarbazine, and vincristine chemotherapy, reduced whole-brain radiotherapy, and local ocular therapy. British journal of haematology. 2012 Apr;
[PubMed PMID: 22085111]
[91]
Hormigo A,Abrey L,Heinemann MH,DeAngelis LM, Ocular presentation of primary central nervous system lymphoma: diagnosis and treatment. British journal of haematology. 2004 Jul;
[PubMed PMID: 15238140]
[92]
Yang XL,Liu YB, Advances in Pathobiology of Primary Central Nervous System Lymphoma. Chinese medical journal. 2017 Aug 20;
[PubMed PMID: 28776551]
Level 3 (low-level) evidence
[94]
Jahnke K,Wagner T,Bechrakis NE,Willerding G,Coupland SE,Fischer L,Thiel E,Korfel A, Pharmacokinetics and efficacy of ifosfamide or trofosfamide in patients with intraocular lymphoma. Annals of oncology : official journal of the European Society for Medical Oncology. 2005 Dec;
[PubMed PMID: 16219622]
[95]
Grimm SA,McCannel CA,Omuro AM,Ferreri AJ,Blay JY,Neuwelt EA,Siegal T,Batchelor T,Jahnke K,Shenkier TN,Hall AJ,Graus F,Herrlinger U,Schiff D,Raizer J,Rubenstein J,Laperriere N,Thiel E,Doolittle N,Iwamoto FM,Abrey LE, Primary CNS lymphoma with intraocular involvement: International PCNSL Collaborative Group Report. Neurology. 2008 Oct 21;
[PubMed PMID: 18936428]